What determines patient preferences for treating low risk basal cell carcinoma when comparing surgery vs imiquimod? A discrete choice experiment survey from the SINS trial
- PMID: 23035730
- PMCID: PMC3532314
- DOI: 10.1186/1471-5945-12-19
What determines patient preferences for treating low risk basal cell carcinoma when comparing surgery vs imiquimod? A discrete choice experiment survey from the SINS trial
Abstract
Background: The SINS trial (Controlled Clinical Trials ISRCTN48755084; Eudract No. 2004-004506-24) is a randomised controlled trial evaluating long term success of excisional surgery vs. imiquimod 5% cream for low risk nodular and superficial basal cell carcinoma (BCC). The trial included a discrete choice experiment questionnaire to explore patient preferences of a cream versus surgery for the treatment of their skin cancer.
Methods: The self-completed questionnaire was administered at baseline to 183 participants, measuring patients' strength of preferences when choosing either alternative 'surgery' or 'imiquimod cream' instead of a fixed 'current situation' option (of surgical excision as standard practice in UK). The treatments were described according to: cost, chance of complete clearance, side effects and appearance. Participants had to choose between various scenarios. Analysis was performed using a mixed logit model, which took into account the impact of previous BCC treatment and sample preference variability.
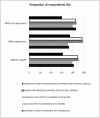
Results: The analysis showed that respondents preferred 'imiquimod cream' to their 'current situation' or 'surgery', regardless of previous experience of BCC symptoms and treatment. Respondents were more likely to be worried about their cosmetic outcomes and side effects they might experience over and above their chance of clearance and cost. Those with no experience of surgery (compared with experience) valued more the choice of 'imiquimod cream' (£1013 vs £781). All treatment characteristics were significant determinants of treatment choice, and there was significant variability in the population preferences for all of them.
Conclusions: Patients with BCC valued more 'imiquimod cream' than alternative 'surgery' options, and all treatment characteristics were important for their choice of care. Understanding how people with a BCC value alternative interventions may better inform the development of health care interventions.
Figures



Similar articles
-
The SINS trial: a randomised controlled trial of excisional surgery versus imiquimod 5% cream for nodular and superficial basal cell carcinoma.Trials. 2010 Apr 21;11:42. doi: 10.1186/1745-6215-11-42. Trials. 2010. PMID: 20409337 Free PMC article. Clinical Trial.
-
Surgical excision versus imiquimod 5% cream for nodular and superficial basal-cell carcinoma (SINS): a multicentre, non-inferiority, randomised controlled trial.Lancet Oncol. 2014 Jan;15(1):96-105. doi: 10.1016/S1470-2045(13)70530-8. Epub 2013 Dec 11. Lancet Oncol. 2014. PMID: 24332516 Clinical Trial.
-
Treatment of cutaneous tumors with topical 5% imiquimod cream.Clinics (Sao Paulo). 2009;64(10):961-6. doi: 10.1590/S1807-59322009001000005. Clinics (Sao Paulo). 2009. PMID: 19841702 Free PMC article.
-
Imiquimod: a review of basal cell carcinoma treatments.J Drugs Dermatol. 2008 Nov;7(11):1044-51. J Drugs Dermatol. 2008. PMID: 19110735 Review.
-
Efficacy and safety of imiquimod 5% cream for basal cell carcinoma: a meta-analysis of randomized controlled trial.J Dermatolog Treat. 2020 Dec;31(8):831-838. doi: 10.1080/09546634.2019.1638883. Epub 2019 Jul 22. J Dermatolog Treat. 2020. PMID: 31294669 Review.
Cited by
-
Respondent Understanding in Discrete Choice Experiments: A Scoping Review.Patient. 2021 Jan;14(1):17-53. doi: 10.1007/s40271-020-00467-y. Epub 2020 Nov 3. Patient. 2021. PMID: 33141359 Free PMC article.
-
Topical Treatments for Basal Cell Carcinoma and Actinic Keratosis in the United States.Cancers (Basel). 2023 Aug 2;15(15):3927. doi: 10.3390/cancers15153927. Cancers (Basel). 2023. PMID: 37568743 Free PMC article. Review.
-
Immunotherapy in Basal Cell Carcinoma.J Clin Med. 2024 Sep 26;13(19):5730. doi: 10.3390/jcm13195730. J Clin Med. 2024. PMID: 39407789 Free PMC article. Review.
-
A Systematic Review of Discrete Choice Experiments in Oncology Treatments.Patient. 2021 Nov;14(6):775-790. doi: 10.1007/s40271-021-00520-4. Epub 2021 May 5. Patient. 2021. PMID: 33950476
-
Sample Size Requirements for Discrete-Choice Experiments in Healthcare: a Practical Guide.Patient. 2015 Oct;8(5):373-84. doi: 10.1007/s40271-015-0118-z. Patient. 2015. PMID: 25726010 Free PMC article. Review.
References
-
- Essers BAB, Van Helvoort-Postulart D, Prins MH. et al.Does the Inclusion of a Cost Attribute Result in Different Preferences for the Surgical Treatment of Primary Basal Cell Carcinoma?: A Comparison of Two Discrete-Choice Experiments. PharmacoEconomics. 2010;28:507–520. doi: 10.2165/11532240-000000000-00000. - DOI - PubMed
-
- Ryan M, Gerard K, Amaya-Amaya M. Using Discrete Choice Experiments to Value Health and Health Care. New York: Springer Berlin Heidelberg; 2008.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

