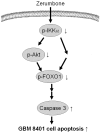
Zerumbone suppresses IKKα, Akt, and FOXO1 activation, resulting in apoptosis of GBM 8401 cells
- PMID: 23035900
- PMCID: PMC3502293
- DOI: 10.1186/1423-0127-19-86
Zerumbone suppresses IKKα, Akt, and FOXO1 activation, resulting in apoptosis of GBM 8401 cells
Abstract
Background: Zerumbone, a sesquiterpene compound isolated from subtropical ginger, Zingiber zerumbet Smith, has been documented to exert antitumoral and anti- inflammatory activities. In this study, we demonstrate that zerumbone induces apoptosis in human glioblastoma multiforme (GBM8401) cells and investigate the apoptotic mechanism.
Methods: We added a caspase inhibitor and transfected wild-type (WT) IKK and Akt into GBM 8401 cells, and measured cell viability and apoptosis by MTT assay and flow cytometry. By western blotting, we evaluated activation of caspase-3, dephosphorylation of IKK, Akt, FOXO1 with time, and change of IKK, Akt, and FOXO1 phosphorylation after transfection of WT IKK and Akt.
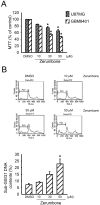
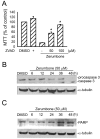
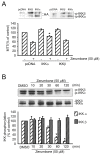
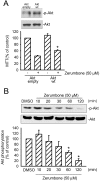
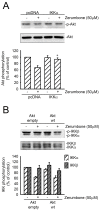
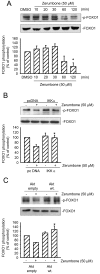
Results: Zerumbone (10~50 μM) induced death of GBM8401 cells in a dose-dependent manner. Flow cytometry studies showed that zerumbone increased the percentage of apoptotic GBM cells. Zerumbone also caused caspase-3 activation and poly (ADP-ribose) polymerase (PARP) production. N-benzyloxycarbonyl -Val-Ala-Asp- fluoromethylketone (zVAD-fmk), a broad-spectrum caspase inhibitor, hindered zerumbone-induced cell death. Transfection of GBM 8401 cells with WT IKKα inhibited zerumbone-induced apoptosis, and zerumbone significantly decreased IKKα phosphorylation levels in a time-dependent manner. Similarly, transfection of GBM8401 cells with Akt suppressed zerumbone-induced apoptosis, and zerumbone also diminished Akt phosphorylation levels remarkably and time-dependently. Moreover, transfection of GBM8401 cells with WT IKKα reduced the zerumbone-induced decrease in Akt and FOXO1 phosphorylation. However, transfection with WT Akt decreased FOXO1, but not IKKα, phosphorylation.
Conclusion: The results suggest that inactivation of IKKα, followed by Akt and FOXO1 phosphorylation and caspase-3 activation, contributes to zerumbone-induced GBM cell apoptosis.
Figures
Similar articles
-
Denbinobin induces human glioblastoma multiforme cell apoptosis through the IKKα-Akt-FKHR signaling cascade.Eur J Pharmacol. 2013 Jan 5;698(1-3):103-9. doi: 10.1016/j.ejphar.2012.10.029. Epub 2012 Oct 30. Eur J Pharmacol. 2013. PMID: 23123054
-
Zerumbone Promotes Cytotoxicity in Human Malignant Glioblastoma Cells through Reactive Oxygen Species (ROS) Generation.Oxid Med Cell Longev. 2020 May 6;2020:3237983. doi: 10.1155/2020/3237983. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32454937 Free PMC article.
-
Zerumbone reduced the inflammatory response of acute lung injury in endotoxin-treated mice via Akt-NFκB pathway.Chem Biol Interact. 2017 Jun 1;271:9-14. doi: 10.1016/j.cbi.2017.04.017. Epub 2017 Apr 22. Chem Biol Interact. 2017. PMID: 28442377
-
Curcumin induces apoptosis in pancreatic cancer cells through the induction of forkhead box O1 and inhibition of the PI3K/Akt pathway.Mol Med Rep. 2015 Oct;12(4):5415-22. doi: 10.3892/mmr.2015.4060. Epub 2015 Jul 8. Mol Med Rep. 2015. PMID: 26166196
-
A Review on zerumbone and its semisynthetic Analogs: Synthesis and Implications in Medicinal chemistry.Bioorg Chem. 2025 Jan;154:108074. doi: 10.1016/j.bioorg.2024.108074. Epub 2024 Dec 24. Bioorg Chem. 2025. PMID: 39732090 Review.
Cited by
-
Binding mode analysis of zerumbone to key signal proteins in the tumor necrosis factor pathway.Int J Mol Sci. 2015 Jan 26;16(2):2747-66. doi: 10.3390/ijms16022747. Int J Mol Sci. 2015. PMID: 25629232 Free PMC article.
-
A novel component from citrus, ginger, and mushroom family exhibits antitumor activity on human meningioma cells through suppressing the Wnt/β-catenin signaling pathway.Tumour Biol. 2015 Sep;36(9):7027-34. doi: 10.1007/s13277-015-3388-0. Epub 2015 Apr 12. Tumour Biol. 2015. PMID: 25864108 Free PMC article.
-
Potential Role of Natural Products to Combat Radiotherapy and Their Future Perspectives.Molecules. 2021 Oct 2;26(19):5997. doi: 10.3390/molecules26195997. Molecules. 2021. Retraction in: Molecules. 2023 Dec 14;28(24):8091. doi: 10.3390/molecules28248091. PMID: 34641542 Free PMC article. Retracted. Review.
-
Protein phosphorylation: A potential target in glioma development.Ibrain. 2022 May 8;8(2):176-189. doi: 10.1002/ibra.12038. eCollection 2022 Summer. Ibrain. 2022. PMID: 37786890 Free PMC article. Review.
-
Advances in nano-delivery of phytochemicals for glioblastoma treatment.Discov Nano. 2024 Dec 24;19(1):216. doi: 10.1186/s11671-024-04172-9. Discov Nano. 2024. PMID: 39718730 Free PMC article. Review.
References
-
- Kitayama T, Okamoto T, Hill RK, Kawai Y, Takahashi S, Yonemori S, Yamamoto Y, Ohe K, Uemura S, Sawada S. Chemistry of Zerumbone. 1. Simplified Isolation, Conjugate Addition Reactions, and a Unique Ring Contracting Transannular Reaction of Its Dibromide. J Org Chem. 1999;64(8):2667–2672. doi: 10.1021/jo981593n. - DOI - PubMed
-
- Murakami A, Takahashi D, Kinoshita T, Koshimizu K, Kim HW, Yoshihiro A, Nakamura Y, Jiwajinda S, Terao J, Ohigashi H. Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: the alpha, beta-unsaturated carbonyl group is a prerequisite. Carcinogenesis. 2002;23(5):795–802. doi: 10.1093/carcin/23.5.795. - DOI - PubMed
-
- Murakami A, Takahashi M, Jiwajinda S, Koshimizu K, Ohigashi H. Identification of zerumbone in Zingiber zerumbet Smith as a potent inhibitor of 12-O-tetradecanoylphorbol-13-acetate-induced Epstein-Barr virus activation. Biosci Biotechnol Biochem. 1999;63(10):1811–1812. doi: 10.1271/bbb.63.1811. - DOI - PubMed
-
- Takada Y, Murakami A, Aggarwal BB. Zerumbone abolishes NF-kappaB and IkappaBalpha kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene. 2005;24(46):6957–6969. doi: 10.1038/sj.onc.1208845. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

