Angiopoietin-1 variant reduces LPS-induced microvascular dysfunction in a murine model of sepsis
- PMID: 23036162
- PMCID: PMC3682284
- DOI: 10.1186/cc11666
Angiopoietin-1 variant reduces LPS-induced microvascular dysfunction in a murine model of sepsis
Abstract
Introduction: Severe sepsis is characterised by intravascular or extravascular infection with microbial agents, systemic inflammation and microcirculatory dysfunction, leading to tissue damage, organ failure and death. The growth factor angiopoietin (Ang-1) has therapeutic potential but recombinant Ang-1 tends to aggregate and has a short half-life in vivo. This study aimed to investigate the acute effects of the more stable Ang-1 variant matrilin-1-angiopoietin-1 (MAT.Ang-1) on the function of the microcirculation in an experimental model of sepsis, and whether any protection by MAT-Ang-1 was associated with modulation of inflammatory cytokines, angiogenic factors or the endothelial nitric oxide synthase (eNOS)-Akt and vascular endothelial (VE)-cadherin pathways.
Methods: Aluminium window chambers were implanted into the dorsal skinfold of male C3H/HeN mice (7 to 10 weeks old) to expose the striated muscle microcirculation. Endotoxemia was induced by intraperitoneal injection of lipopolysaccharide (LPS, 1 mg/kg at 0 and 19 hours). MAT.Ang-1 was administered intravenously 20 hours after the onset of sepsis. Microcirculatory function was evaluated by intravital microscopy and Doppler fluximetry.
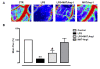
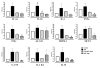
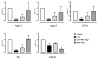
Results: Endotoxemia resulted in macromolecular leak, which was ameliorated by MAT.Ang-1 post-treatment. LPS induced a dramatic reduction in tissue perfusion, which was improved by MAT.Ang-1. Proteome profiler array analysis of skeletal muscle also demonstrated increased inflammatory and reduced angiogenic factors during endotoxemia. MAT.Ang-1 post-treatment reduced the level of IL-1β but did not significantly induce the expression of angiogenic factors. MAT.Ang-1 alone did not induce leak or increase angiogenic factors but did reduce vascular endothelial growth factor expression in controls.
Conclusion: Administration of MAT.Ang-1 after the onset of sepsis protects the microcirculation from endotoxemia-induced vascular dysfunction through reducing inflammation but without pro-angiogenic actions, thus representing a novel, potential pharmacotherapeutic agent for the treatment of sepsis.
Figures

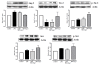

Comment in
-
Fixing the leak: targeting the vascular endothelium in sepsis.Crit Care. 2012 Nov 21;16(6):177. doi: 10.1186/cc11829. Crit Care. 2012. PMID: 23171759 Free PMC article.
-
Unraveling the mechanisms involved in endothelial barrier protective effects of angiopoietin-1 variant MAT.Ang-1.Crit Care. 2012 Nov 27;16(6):466; author reply 466. doi: 10.1186/cc11844. Crit Care. 2012. PMID: 23186009 Free PMC article. No abstract available.
Similar articles
-
Angiopoietin-1 regulates microvascular reactivity and protects the microcirculation during acute endothelial dysfunction: role of eNOS and VE-cadherin.Pharmacol Res. 2014 Feb;80:43-51. doi: 10.1016/j.phrs.2013.12.008. Epub 2014 Jan 7. Pharmacol Res. 2014. PMID: 24407281
-
Unraveling the mechanisms involved in endothelial barrier protective effects of angiopoietin-1 variant MAT.Ang-1.Crit Care. 2012 Nov 27;16(6):466; author reply 466. doi: 10.1186/cc11844. Crit Care. 2012. PMID: 23186009 Free PMC article. No abstract available.
-
Fixing the leak: targeting the vascular endothelium in sepsis.Crit Care. 2012 Nov 21;16(6):177. doi: 10.1186/cc11829. Crit Care. 2012. PMID: 23171759 Free PMC article.
-
Angiopoietin-1 in the treatment of ischemia and sepsis.Shock. 2009 Apr;31(4):335-41. doi: 10.1097/SHK.0b013e3181862c63. Shock. 2009. PMID: 18791498 Review.
-
Microcirculatory alterations in ischemia-reperfusion injury and sepsis: effects of activated protein C and thrombin inhibition.Crit Care. 2005;9 Suppl 4(Suppl 4):S33-7. doi: 10.1186/cc3758. Epub 2005 Aug 25. Crit Care. 2005. PMID: 16168073 Free PMC article. Review.
Cited by
-
Vasculotide, an Angiopoietin-1 mimetic, ameliorates several features of experimental atopic dermatitis-like disease.BMC Res Notes. 2016 May 28;9:289. doi: 10.1186/s13104-015-1817-1. BMC Res Notes. 2016. PMID: 27236199 Free PMC article.
-
Beyond single-nucleotide polymorphisms: genetics, genomics, and other 'omic approaches to acute respiratory distress syndrome.Clin Chest Med. 2014 Dec;35(4):673-84. doi: 10.1016/j.ccm.2014.08.006. Epub 2014 Sep 23. Clin Chest Med. 2014. PMID: 25453417 Free PMC article. Review.
-
In Development-A New Paradigm for Understanding Vascular Disease.J Cardiovasc Pharmacol. 2017 May;69(5):248-263. doi: 10.1097/FJC.0000000000000480. J Cardiovasc Pharmacol. 2017. PMID: 28328747 Free PMC article. Review.
-
The Endothelial Glycocalyx: A Fundamental Determinant of Vascular Permeability in Sepsis.Pediatr Crit Care Med. 2020 May;21(5):e291-e300. doi: 10.1097/PCC.0000000000002266. Pediatr Crit Care Med. 2020. PMID: 32132499 Free PMC article. Review.
-
Angiogenesis-related biomarkers in patients with alcoholic liver disease: their association with liver disease complications and outcome.Mediators Inflamm. 2014;2014:673032. doi: 10.1155/2014/673032. Epub 2014 May 18. Mediators Inflamm. 2014. PMID: 24959006 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

