Cancer nanomedicines: so many papers and so few drugs!
- PMID: 23036224
- PMCID: PMC3565003
- DOI: 10.1016/j.addr.2012.09.038
Cancer nanomedicines: so many papers and so few drugs!
Abstract
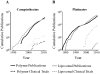
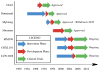
This review identifies a timeline to nanomedicine anticancer drug approval using the business model of inventors, innovators and imitators. By evaluating the publication record of nanomedicine cancer therapeutics we identified a trend of very few publications prior to FDA approval. We first enumerated the publications related to cancer involving polymers, liposomes or monoclonal antibodies and determined the number of citations per publication as well as the number of published clinical trials among the publications. Combining these data with the development of specific nanomedicines, we are able to identify an invention phase consisting of seminal papers in basic science necessary for the development of a specific nanomedicine. The innovation phase includes the first report, the development and the clinical trials involving that nanomedicine. Finally, the imitation phase begins after approval when others ride the wave of success by using the same formulation for new drugs or using the same drug to validate other nanomedicines. We then focused our analysis on nanomedicines containing camptothecin derivatives, which are not yet approved including two polymers considered innovations and one liposomal formulation in the imitation phase. The conclusion that may be drawn from the analysis of the camptothecins is that approved drugs reformulated in polymeric and liposomal cancer nanomedicines have a more difficult time navigating through the approval process than the parent molecule. This is probably due to the fact that for most currently approved drugs, reformulating them in a nanocarrier provides a small increase in performance that large pharmaceutical companies do not consider being worth the time, effort and expense of development. It also appears that drug carriers have a more difficult path through the clinic than monoclonal antibodies. The added complexity of nanocarriers also deters their use to deliver new molecular entities. Thus, the new drug candidates that might be most improved by drug delivery in nanocarriers are not formulated in this fashion.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
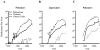

Similar articles
-
Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
-
Clinical developments of chemotherapeutic nanomedicines: polymers and liposomes for delivery of camptothecins and platinum (II) drugs.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013 Mar-Apr;5(2):130-8. doi: 10.1002/wnan.1209. Epub 2013 Jan 17. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013. PMID: 23335566 Review.
-
Nanomedicines for advanced cancer treatments: Transitioning towards responsive systems.Int J Pharm. 2016 Dec 30;515(1-2):132-164. doi: 10.1016/j.ijpharm.2016.10.013. Epub 2016 Oct 7. Int J Pharm. 2016. PMID: 27725268 Review.
-
Liposomal nanomedicines.Expert Opin Drug Deliv. 2008 Jan;5(1):25-44. doi: 10.1517/17425247.5.1.25. Expert Opin Drug Deliv. 2008. PMID: 18095927 Review.
-
Receptor-based targeting of engineered nanocarrier against solid tumors: Recent progress and challenges ahead.Biochim Biophys Acta Gen Subj. 2021 Feb;1865(2):129777. doi: 10.1016/j.bbagen.2020.129777. Epub 2020 Oct 29. Biochim Biophys Acta Gen Subj. 2021. PMID: 33130062 Review.
Cited by
-
Solid Phase Extraction as an Innovative Separation Method for Measuring Free and Entrapped Drug in Lipid Nanoparticles.Pharm Res. 2015 Dec;32(12):3999-4009. doi: 10.1007/s11095-015-1761-8. Epub 2015 Jul 23. Pharm Res. 2015. PMID: 26202518
-
Nanomedicine: is the wave cresting?Nat Rev Drug Discov. 2013 Mar;12(3):171-2. doi: 10.1038/nrd3958. Nat Rev Drug Discov. 2013. PMID: 23449291 Free PMC article. No abstract available.
-
In vivo Functional Evaluation of Increased Brain Delivery of the Opioid Peptide DAMGO by Glutathione-PEGylated Liposomes.Pharm Res. 2016 Jan;33(1):177-85. doi: 10.1007/s11095-015-1774-3. Epub 2015 Aug 15. Pharm Res. 2016. PMID: 26275529
-
Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances.Mater Today (Kidlington). 2016 Apr;19(3):157-168. doi: 10.1016/j.mattod.2015.08.022. Mater Today (Kidlington). 2016. PMID: 27524934 Free PMC article.
-
Transferrin-Conjugated Docetaxel-PLGA Nanoparticles for Tumor Targeting: Influence on MCF-7 Cell Cycle.Polymers (Basel). 2019 Nov 19;11(11):1905. doi: 10.3390/polym11111905. Polymers (Basel). 2019. PMID: 31752417 Free PMC article.
References
-
- Feynman RP. There’s plenty of room at the bottom. Eng Sci (CalTech) 1960;23:22–36.
-
- Sarker D, Workman P. Pharmacodynamic biomarkers for molecular cancer therapeutics. Adv Cancer Res. 2007;96:213–268. - PubMed
-
- Alexis F, Pridgen EM, Langer R, Farokhzad OC. Nanoparticle technologies for cancer therapy. In: Schafer-Korting M, editor. Drug Delivery, Handbook of Experimental Pharmacology. Vol. 197. Springer-Verlag; Berlin: 2010. pp. 55–86. - PubMed
-
- Duncan R, Gaspar R. Nanomedicine(s) under the microscope. Mol Pharmaceut. 2011;8:2101–2141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

