The posterior hypothalamus exerts opposing effects on nociception via the A7 catecholamine cell group in rats
- PMID: 23036619
- PMCID: PMC3932022
- DOI: 10.1016/j.neuroscience.2012.09.058
The posterior hypothalamus exerts opposing effects on nociception via the A7 catecholamine cell group in rats
Abstract
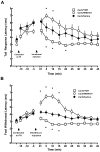
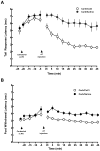
Stimulation of the posterior hypothalamic area (PH) produces antinociception in rats and humans, but the precise mechanisms are unknown. The PH forms anatomical connections with the parabrachial area, which contains the pontine A7 catecholamine cell group, a group of spinally projecting noradrenergic neurons known to produce antinociception in the dorsal horn. The aim of the present study was to determine whether PH-induced antinociception is mediated in part through connections with the A7 cell group in female Sprague-Dawley rats, as measured by the tail flick and foot withdrawal latency. Stimulation of the PH with the cholinergic agonist carbachol (125 nmol) produced antinociception that was blocked by pretreatment with atropine sulfate. Intrathecal injection of the α(2)-adrenoceptor antagonist yohimbine reversed PH-induced antinociception, but the α(1)-adrenoceptor antagonist WB4101 facilitated antinociception. Intrathecal injection of normal saline had no effect. In a separate experiment, cobalt chloride, which reversibly arrests synaptic activity, was microinjected into the A7 cell group and blocked PH-induced antinociception. These findings provide evidence that the PH modulates nociception in part through connections with the A7 catecholamine cell group through opposing effects. Antinociception occurs from actions at α(2)-adrenoceptors in the dorsal horn, while concurrent hyperalgesia occurs from actions of norepinephrine at α(1)-adrenoceptors. This hyperalgesic response likely attenuates antinociception from PH stimulation.
Copyright © 2012 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Abrahamson E, Moore R. The posterior hypothalamic area: Chemoarchitecture and afferent connections. Brain Res. 2001;889:1–22. - PubMed
-
- Bartsch T, Falk D, et al. Deep brain stimulation of the posterior hypothalamic area in intractable short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT) Cephalgia. 2011;13:1405–1408. - PubMed
-
- Bartsch T, Levy MJ, et al. Differential modulation of nociceptive dural input to [hypocretin] orexin A and B receptor activation in the posterior hypothalamic area. Pain. 2004;109:367–378. - PubMed
-
- Bartsch T, Pinsker MO, et al. Hypothalamic deep brain stimulation for cluster headache: Experience from a new multicase series. Cephalalgia. 2008;28:285–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

