Lysophosphatidic acid induces increased BACE1 expression and Aβ formation
- PMID: 23036978
- PMCID: PMC3518696
- DOI: 10.1016/j.bbadis.2012.09.010
Lysophosphatidic acid induces increased BACE1 expression and Aβ formation
Abstract
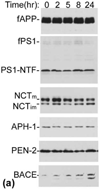
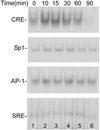
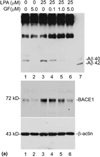
The abnormal production and accumulation of β-amyloid peptide (Aβ), which is produced from amyloid precursor protein (APP) by the sequential actions of β-secretase and γ-secretase, are thought to be the initial causative events in the development of Alzheimer's disease (AD). Accumulating evidence suggests that vascular factors play an important role in the pathogenesis of AD. Specifically, studies have suggested that one vascular factor in particular, oxidized low density lipoprotein (oxLDL), may play an important role in regulating Aβ formation in AD. However, the mechanism by which oxLDL modulates Aβ formation remains elusive. In this study, we report several new findings that provide biochemical evidence suggesting that the cardiovascular risk factor oxLDL may contribute to Alzheimer's disease by increasing Aβ production. First, we found that lysophosphatidic acid (LPA), the most bioactive component of oxLDL induces increased production of Aβ. Second, our data strongly indicate that LPA induces increased Aβ production via upregulating β-secretase expression. Third, our data strongly support the notion that different isoforms of protein kinase C (PKC) may play different roles in regulating APP processing. Specifically, most PKC members, such as PKCα, PKCβ, and PKCε, are implicated in regulating α-secretase-mediated APP processing; however, PKCδ, a member of the novel PKC subfamily, is involved in LPA-induced upregulation of β-secretase expression and Aβ production. These findings may contribute to a better understanding of the mechanisms by which the cardiovascular risk factor oxLDL is involved in Alzheimer's disease.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures












References
-
- Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. Amyloid-beta oligomers: their production, toxicity and therapeutic inhibition. Biochem. Soc. Trans. 2002;30:552–557. - PubMed
-
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. - PubMed
-
- Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, Carter DB, Tomasselli AG, Parodi LA, Heinrikson RL, Gurney ME. Membrane-anchored aspartyl protease with Alzheimer's disease beta-secretase activity. Nature. 1999;402:533–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

