Production of selenoprotein P (Sepp1) by hepatocytes is central to selenium homeostasis
- PMID: 23038251
- PMCID: PMC3504756
- DOI: 10.1074/jbc.M112.421404
Production of selenoprotein P (Sepp1) by hepatocytes is central to selenium homeostasis
Abstract
Background: Sepp1 transports selenium, but its complete role in selenium homeostasis is not known.
Results: Deletion of Sepp1 in hepatocytes increases liver selenium at the expense of other tissues and decreases whole-body selenium by increasing excretion.
Conclusion: Sepp1 production by hepatocytes retains selenium in the organism and distributes it from the liver to peripheral tissues.
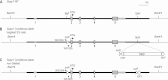
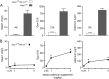
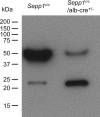
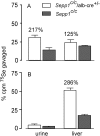
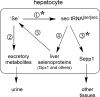
Significance: Sepp1 is central to selenium homeostasis. Sepp1 is a widely expressed extracellular protein that in humans and mice contains 10 selenocysteine residues in its primary structure. Extra-hepatic tissues take up plasma Sepp1 for its selenium via apolipoprotein E receptor-2 (apoER2)-mediated endocytosis. The role of Sepp1 in the transport of selenium from liver, a rich source of the element, to peripheral tissues was studied using mice with selective deletion of Sepp1 in hepatocytes (Sepp1(c/c)/alb-cre(+/-) mice). Deletion of Sepp1 in hepatocytes lowered plasma Sepp1 concentration to 10% of that in Sepp1(c/c) mice (controls) and increased urinary selenium excretion, decreasing whole-body and tissue selenium concentrations. Under selenium-deficient conditions, Sepp1(c/c)/alb-cre(+/-) mice accumulated selenium in the liver at the expense of extra-hepatic tissues, severely worsening clinical manifestations of dietary selenium deficiency. These findings are consistent with there being competition for metabolically available hepatocyte selenium between the synthesis of selenoproteins and the synthesis of selenium excretory metabolites. In addition, selenium deficiency down-regulated the mRNA of the most abundant hepatic selenoprotein, glutathione peroxidase-1 (Gpx1), to 15% of the selenium-replete value, while reducing Sepp1 mRNA, the most abundant hepatic selenoprotein mRNA, only to 61%. This strongly suggests that Sepp1 synthesis is favored in the liver over Gpx1 synthesis when selenium supply is limited, directing hepatocyte selenium to peripheral tissues in selenium deficiency. We conclude that production of Sepp1 by hepatocytes is central to selenium homeostasis in the organism because it promotes retention of selenium in the body and effects selenium distribution from the liver to extra-hepatic tissues, especially under selenium-deficient conditions.
Figures




References
-
- Kryukov G. V., Castellano S., Novoselov S. V., Lobanov A. V., Zehtab O., Guigó R., Gladyshev V. N. (2003) Characterization of mammalian selenoproteomes. Science 300, 1439–1443 - PubMed
-
- Ma S., Hill K. E., Caprioli R. M., Burk R. F. (2002) Mass spectrometric characterization of full-length rat selenoprotein P and three isoforms shortened at the C terminus. Evidence that three UGA codons in the mRNA open reading frame have alternative functions of specifying selenocysteine insertion or translation termination. J. Biol. Chem. 277, 12749–12754 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

