Inhibitory glycine receptors: an update
- PMID: 23038260
- PMCID: PMC3504737
- DOI: 10.1074/jbc.R112.408229
Inhibitory glycine receptors: an update
Abstract
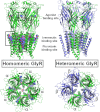
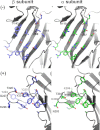
Strychnine-sensitive glycine receptors (GlyRs) mediate synaptic inhibition in the spinal cord, brainstem, and other regions of the mammalian central nervous system. In this minireview, we summarize our current view of the structure, ligand-binding sites, and chloride channel of these receptors and discuss recently emerging functions of distinct GlyR isoforms. GlyRs not only regulate the excitability of motor and afferent sensory neurons, including pain fibers, but also are involved in the processing of visual and auditory signals. Hence, GlyRs constitute promising targets for the development of therapeutically useful compounds.
Figures

References
-
- Pfeiffer F., Graham D., Betz H. (1982) Purification by affinity chromatography of the glycine receptor of rat spinal cord. J. Biol. Chem. 257, 9389–9393 - PubMed
-
- Betz H., Laube B. (2006) Glycine receptors: recent insights into their structural organization and functional diversity. J. Neurochem. 97, 1600–1610 - PubMed
-
- Lynch J. W. (2004) Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev. 84, 1051–1095 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

