Ciliary neurotrophic factor protects mice against streptozotocin-induced type 1 diabetes through SOCS3: the role of STAT1/STAT3 ratio in β-cell death
- PMID: 23038263
- PMCID: PMC3516714
- DOI: 10.1074/jbc.M112.358788
Ciliary neurotrophic factor protects mice against streptozotocin-induced type 1 diabetes through SOCS3: the role of STAT1/STAT3 ratio in β-cell death
Abstract
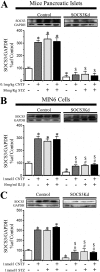
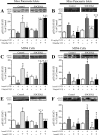
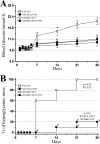
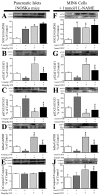
Type 1 diabetes is characterized by a loss of islet β-cells. Ciliary neurotrophic factor (CNTF) protects pancreatic islets against cytokine-induced apoptosis. For this reason, we assessed whether CNTF protects mice against streptozotocin-induced diabetes (a model of type 1 diabetes) and the mechanism for this protection. WT and SOCS3 knockdown C57BL6 mice were treated for 5 days with citrate buffer or 0.1 mg/kg CNTF before receiving 80 mg/kg streptozotocin. Glycemia in non-fasted mice was measured weekly from days 0-28 after streptozotocin administration. Diabetes was defined as a blood glucose > 11.2 mmol/liter. Wild-type (WT) and SOCS3 knockdown MIN6 cells were cultured with CNTF, IL1β, or both. CNTF reduced diabetes incidence and islet apoptosis in WT but not in SOCS3kd mice. Likewise, CNTF inhibited apoptosis in WT but not in SOCS3kd MIN6 cells. CNTF increased STAT3 phosphorylation in WT and SOCS3kd mice and MIN6 cells but reduced STAT1 phosphorylation only in WT mice, in contrast to streptozotocin and IL1β. Moreover, CNTF reduced NFκB activation and required down-regulation of inducible NO synthase expression to exert its protective effects. In conclusion, CNTF protects mice against streptozotocin-induced diabetes by increasing pancreatic islet survival, and this protection depends on SOCS3. In addition, SOCS3 expression and β-cell fate are dependent on STAT1/STAT3 ratio.
Figures




Similar articles
-
Ciliary neurotrophic factor (CNTF) signals through STAT3-SOCS3 pathway and protects rat pancreatic islets from cytokine-induced apoptosis.Cytokine. 2009 Apr;46(1):65-71. doi: 10.1016/j.cyto.2008.12.014. Epub 2009 Mar 9. Cytokine. 2009. PMID: 19272793
-
CNTF protects MIN6 cells against apoptosis induced by Alloxan and IL-1β through downregulation of the AMPK pathway.Cell Signal. 2011 Oct;23(10):1669-76. doi: 10.1016/j.cellsig.2011.06.001. Epub 2011 Jun 15. Cell Signal. 2011. PMID: 21689743
-
Ciliary neurotrophic factor (CNTF) protects non-obese Swiss mice against type 2 diabetes by increasing beta cell mass and reducing insulin clearance.Diabetologia. 2012 May;55(5):1495-504. doi: 10.1007/s00125-012-2493-5. Epub 2012 Feb 19. Diabetologia. 2012. PMID: 22349107
-
Suppression of SOCS3 expression in the pancreatic beta-cell leads to resistance to type 1 diabetes.Biochem Biophys Res Commun. 2007 Aug 10;359(4):952-8. doi: 10.1016/j.bbrc.2007.05.198. Epub 2007 Jun 4. Biochem Biophys Res Commun. 2007. PMID: 17562326
-
The Roles of Ciliary Neurotrophic Factor - from Neuronutrition to Energy Metabolism.Protein Pept Lett. 2022;29(10):815-828. doi: 10.2174/0929866529666220905105800. Protein Pept Lett. 2022. PMID: 36065930 Review.
Cited by
-
Increased Expression of Circulating microRNA 101-3p in Type 1 Diabetes Patients: New Insights Into miRNA-Regulated Pathophysiological Pathways for Type 1 Diabetes.Front Immunol. 2019 Jul 23;10:1637. doi: 10.3389/fimmu.2019.01637. eCollection 2019. Front Immunol. 2019. PMID: 31396209 Free PMC article.
-
Lycopene ameliorates diabetes-induced pancreatic, hepatic, and renal damage by modulating the JAK/STAT/SOCS signaling pathway in rats.Iran J Basic Med Sci. 2025;28(4):461-468. doi: 10.22038/ijbms.2025.79979.17326. Iran J Basic Med Sci. 2025. PMID: 39968090 Free PMC article.
-
Cardiotrophin-1 (CT-1) improves high fat diet-induced cognitive deficits in mice.Neurochem Res. 2015 Apr;40(4):843-53. doi: 10.1007/s11064-015-1535-z. Epub 2015 Feb 12. Neurochem Res. 2015. PMID: 25672823
-
The Impact of Psilocybin on High Glucose/Lipid-Induced Changes in INS-1 Cell Viability and Dedifferentiation.Genes (Basel). 2024 Jan 29;15(2):183. doi: 10.3390/genes15020183. Genes (Basel). 2024. PMID: 38397173 Free PMC article.
-
Ciliary neurotrophic factor is increased in the plasma of patients with obesity and its levels correlate with diabetes and inflammation indices.Sci Rep. 2022 May 18;12(1):8331. doi: 10.1038/s41598-022-11942-x. Sci Rep. 2022. PMID: 35585213 Free PMC article.
References
-
- Gysemans C. A., Ladrière L., Callewaert H., Rasschaert J., Flamez D., Levy D. E., Matthys P., Eizirik D. L., Mathieu C. (2005) Disruption of the γ-interferon signaling pathway at the level of signal transducer and activator of transcription-1 prevents immune destruction of β-cells. Diabetes 54, 2396–2403 - PubMed
-
- Cardozo A. K., Heimberg H., Heremans Y., Leeman R., Kutlu B., Kruhøffer M., Ørntoft T., Eizirik D. L. (2001) A comprehensive analysis of cytokine-induced and nuclear factor-κB-dependent genes in primary rat pancreatic β-cells. J. Biol. Chem. 276, 48879–48886 - PubMed
-
- Eizirik D. L., Mandrup-Poulsen T. (2001) A choice of death–the signal transduction of immune-mediated β-cell apoptosis. Diabetologia 44, 2115–2133 - PubMed
-
- Cnop M., Welsh N., Jonas J. C., Jörns A., Lenzen S., Eizirik D. L. (2005) Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54, S97–107 - PubMed
-
- Kishimoto T., Akira S., Narazaki M., Taga T. (1995) Interleukin-6 family of cytokines and gp130. Blood 86, 1243–1254 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

