Structure, function, and modulation of GABA(A) receptors
- PMID: 23038269
- PMCID: PMC3504738
- DOI: 10.1074/jbc.R112.386664
Structure, function, and modulation of GABA(A) receptors
Abstract
The GABA(A) receptors are the major inhibitory neurotransmitter receptors in mammalian brain. Each isoform consists of five homologous or identical subunits surrounding a central chloride ion-selective channel gated by GABA. How many isoforms of the receptor exist is far from clear. GABA(A) receptors located in the postsynaptic membrane mediate neuronal inhibition that occurs in the millisecond time range; those located in the extrasynaptic membrane respond to ambient GABA and confer long-term inhibition. GABA(A) receptors are responsive to a wide variety of drugs, e.g. benzodiazepines, which are often used for their sedative/hypnotic and anxiolytic effects.
Figures

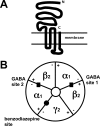


References
-
- Pfeiffer F., Graham D., Betz H. (1982) Purification by affinity chromatography of the glycine receptor of rat spinal cord. J. Biol. Chem. 257, 9389–9393 - PubMed
-
- Sigel E., Mamalaki C., Barnard E. A. (1982) Isolation of a GABA receptor from bovine brain using a benzodiazepine affinity column. FEBS Lett. 147, 45–48 - PubMed
-
- Sigel E., Stephenson F. A., Mamalaki C., Barnard E. A. (1983) A γ-aminobutyric acid/benzodiazepine receptor complex of bovine cerebral cortex. J. Biol. Chem. 258, 6965–6971 - PubMed
-
- Sigel E., Barnard E. A. (1984) A γ-aminobutyric acid/benzodiazepine receptor complex from bovine cerebral cortex. Improved purification with preservation of regulatory sites and their interactions. J. Biol. Chem. 259, 7219–7223 - PubMed
-
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A., Seeburg P. H., Barnard E. A. (1987) Sequence and functional expression of the GABAA receptor shows a ligand-gated receptor superfamily. Nature 328, 221–227 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

