Post-weaning increases in the milk-fat globule EGF-factor VIII on fat globules in mouse milk and in the uptake of the fat globules by HC11 mammary epithelial cells
- PMID: 23038672
- PMCID: PMC3527995
- DOI: 10.1093/jb/mvs116
Post-weaning increases in the milk-fat globule EGF-factor VIII on fat globules in mouse milk and in the uptake of the fat globules by HC11 mammary epithelial cells
Abstract
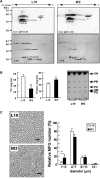
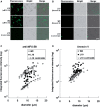
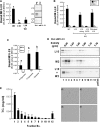
Milk fat globules (MFGs) secreted by lactating mammary gland are unique lipid surrounded by a phospholipid bi-layer. We report here post-weaning changes in MFG EGF factor VIII (MFG-E8) and annexin V-accessible phosphatidyl-l-serine on the surface of MFGs. The MFG content in milk markedly decreased to about one-half within 2 days after forced weaning, despite a slight increase in milk protein content. Immunofluorescence-staining of MFGs using anti-MFG-E8 and annexin V indicated that MFG-E8 was present on some, but not all, MFGs before weaning, whereas most of MFGs were MFG-E8-positive and annexin V-negative after weaning. Free MFG-E8 with binding activity to phosphatidyl-l-serine was present abundantly in the post-weaning milk, and indeed exhibited binding to MFGs in pre-weaning milk. MFGs were taken up by HC11 mouse mammary epithelial cells in vitro, and those from post-weaning milk were remarkable for such cellular uptake. Moreover, the uptake of MFGs by the cells was inhibited by an anti-MFG-E8 antibody. Taken together, these findings suggest that MFG-E8 plays a critical role in regulation of MFG dynamics after weaning or during the suckling interval through the control of MFG-epithelial cell interaction in lactating mammary glands.
Figures


Similar articles
-
Weaning-induced expression of a milk-fat globule protein, MFG-E8, in mouse mammary glands, as demonstrated by the analyses of its mRNA, protein and phosphatidylserine-binding activity.Biochem J. 2006 Apr 1;395(1):21-30. doi: 10.1042/BJ20051459. Biochem J. 2006. PMID: 16401186 Free PMC article.
-
Re-evaluation of milk-fat globule EGF-factor VIII (MFG-E8) as an intrinsic component of the mouse milk-fat globule membrane.Biosci Biotechnol Biochem. 2012;76(11):2055-60. doi: 10.1271/bbb.120412. Epub 2012 Nov 7. Biosci Biotechnol Biochem. 2012. PMID: 23132565
-
A protective effect of milk fat globule EGF factor VIII (MFG-E8) on the spontaneous fusion of milk fat globules in breast milk.J Biochem. 2015 Jul;158(1):25-35. doi: 10.1093/jb/mvv016. Epub 2015 Feb 7. J Biochem. 2015. PMID: 25661589
-
Regulation and functional relevance of milk fat globules and their components in the mammary gland.Biosci Biotechnol Biochem. 2006 Sep;70(9):2019-27. doi: 10.1271/bbb.60142. Epub 2006 Sep 7. Biosci Biotechnol Biochem. 2006. Retraction in: Biosci Biotechnol Biochem. 2013;77(12):2013R3. PMID: 16960354 Retracted. Review.
-
Disorder in milk proteins: adipophilin and TIP47, important constituents of the milk fat globule membrane.J Biomol Struct Dyn. 2020 Mar;38(4):1214-1229. doi: 10.1080/07391102.2019.1592027. Epub 2019 Mar 21. J Biomol Struct Dyn. 2020. PMID: 30896308 Review.
Cited by
-
Characterization and comparison of human and mouse milk cells.PLoS One. 2024 Jan 31;19(1):e0297821. doi: 10.1371/journal.pone.0297821. eCollection 2024. PLoS One. 2024. PMID: 38295101 Free PMC article.
References
-
- Blasiole DA, Davis RA, Attie AD. The physiological and molecular regulation of lipoprotein assembly and secretion. Mol. Biosyst. 2007;3:608–619. - PubMed
-
- Monks J, Geske FJ, Lehman L, Fadok VA. Do inflammatory cells participate in mammary gland involution? J. Mammary Gland Biol. Neoplasia. 2002;7:163–176. - PubMed
-
- Nakatani H, Aoki N, Nakagawa Y, Jin-No S, Aoyama K, Oshima K, Ohira S, Sato C, Nadano D, Matsuda T. Weaning-induced expression of a milk-fat globule protein, MFG-E8, in mouse mammary glands, as demonstrated by the analyses of its mRNA, protein and phosphatidylserine-binding activity. Biochem. J. 2006;395:21–30. - PMC - PubMed
-
- Oshima K, Aoki N, Negi M, Kishi M, Kitajima K, Matsuda T. Lactation-dependent expression of an mRNA splice variant with an exon for a multiply O-glycosylated domain of mouse milk fat globule glycoprotein MFG-E8. Biochem. Biophys. Res. Commun. 1999;254:522–528. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

