The Scribble/Lgl/Dlg polarity protein complex is a regulator of blood-testis barrier dynamics and spermatid polarity during spermatogenesis
- PMID: 23038739
- PMCID: PMC3512062
- DOI: 10.1210/en.2012-1670
The Scribble/Lgl/Dlg polarity protein complex is a regulator of blood-testis barrier dynamics and spermatid polarity during spermatogenesis
Abstract
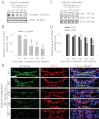
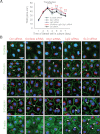
During spermatogenesis, spermiogenesis that releases sperm into the tubule lumen and restructuring of the blood-testis barrier (BTB) that accommodates the transit of preleptotene spermatocytes take place simultaneously, but at the opposite ends of the seminiferous epithelium. These events are tightly regulated and coordinated; however, neither the underlying mechanism(s) nor the involving molecules are known. Herein, the Scribble/Lgl (Lethal giant larvae)/Dlg (Discs large) polarity complex was shown to regulate spermatid polarity during spermiogenesis and tight junction (TJ)-permeability barrier via changes in protein distribution at the apical ectoplasmic specialization and the BTB during the epithelial cycle, respectively. Scribble, Lgl2, and Dlg1 were found to be expressed by Sertoli and germ cells. Scribble also displayed stage-specific expression at the BTB, being highest at stages VII-VIII, colocalizing with TJ proteins occludin and ZO-1. Unlike components of other polarity complex modules, such as partitioning-defective 6, the knockdown of which by RNA interference was found to impede Sertoli cell TJ barrier, a knockdown of the Scribble complex (i.e. simultaneous knockdown of Scribble, Lgl and Dlg or Lgl alone; but not Scribble or Dlg alone) both in vitro and in vivo promoted the TJ integrity. This was mediated by reorganizing actin filament network at the Sertoli cell-cell interface, which, in turn, affected changes in the localization and/or distribution of occludin and/or β-catenin at the BTB. These knockdowns also perturbed F-actin organization at the Sertoli cell-spermatid interface, thereby modulating spermatid adhesion and polarity at the apical ectoplasmic specialization. In summary, the Scribble/Lgl/Dlg complex participates in the regulation of BTB dynamics and spermatid adhesion/polarity in the testis.
Figures





References
-
- Hess RA, de Franca L. 2008. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol 636:1–15 - PubMed

