Drosophila embryos close epithelial wounds using a combination of cellular protrusions and an actomyosin purse string
- PMID: 23038780
- PMCID: PMC3585516
- DOI: 10.1242/jcs.109066
Drosophila embryos close epithelial wounds using a combination of cellular protrusions and an actomyosin purse string
Abstract
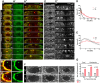
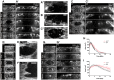
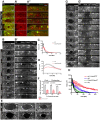
The repair of injured tissue must occur rapidly to prevent microbial invasion and maintain tissue integrity. Epithelial tissues in particular, which serve as a barrier against the external environment, must repair efficiently in order to restore their primary function. Here we analyze the effect of different parameters on the epithelial wound repair process in the late stage Drosophila embryo using in vivo wound assays, expression of cytoskeleton and membrane markers, and mutant analysis. We define four distinct phases in the repair process, expansion, coalescence, contraction and closure, and describe the molecular dynamics of each phase. Specifically, we find that myosin, E-cadherin, Echinoid, the plasma membrane, microtubules and the Cdc42 small GTPase respond dynamically during wound repair. We demonstrate that perturbations of each of these components result in specific impairments to the wound healing process. Our results show that embryonic epithelial wound repair is mediated by two simultaneously acting mechanisms: crawling driven by cellular protrusions and actomyosin ring contraction along the leading edge of the wound.
Figures




References
-
- Buck R. C. (1979). Cell migration in repair of mouse corneal epithelium. Invest. Ophthalmol. Vis. Sci. 18, 767–784 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

