Vaginal ring delivery of selective progesterone receptor modulators for contraception
- PMID: 23040126
- PMCID: PMC3657703
- DOI: 10.1016/j.contraception.2012.08.038
Vaginal ring delivery of selective progesterone receptor modulators for contraception
Abstract
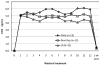
Vaginal ring delivery of selective progesterone receptor modulators (SPRMs) is under development to address the limitations of current hormonal methods that affect use and effectiveness. This method would be appropriate for use in women with contraindications to, or preferences to avoid, estrogens. A contraceptive vaginal ring (CVR) also eliminates the need for daily dosing and therefore might improve the effectiveness of contraception. The principal contraceptive effect of SPRMs is the suppression of ovulation. One limiting factor of chronic SPRM administration is the development of benign endometrial thickening characterized as PRM-associated endometrial changes. Ulipristal acetate (UPA) is approved for use as an emergency contraceptive pill, but no SPRM is approved for regular contraception. The Population Council is developing an ulipristal acetate CVR for regular contraception. The CVR studied is of a matrix design composed of micronized UPA mixed in a silicone rubber matrix The target product is a ring designed for continuous use over 3 months delivering near steady-state drug levels that will suppress ovulation. Results from Phase 1 and 2 studies demonstrate that suppression of ovulation occurs with UPA levels above 6-7 ng/mL.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

References
-
- Curtis KM, Jamieson DJ, Peterson HB, Marchbanks PA. Adaptation of the World Health Organization’s medical eligibility criteria for contraceptive use for use in the United States. Contraception. 2010;82:3–9. - PubMed
-
- Dieben TO, Roumen FJ, Apter D. Efficacy, cycle control, and user acceptability of a novel combined contraceptive vaginal ring. Obstet Gynecol. 2002;100:585–93. - PubMed
-
- Clinical Study report. Protocol 300A/B. New York, New York: Population Council; 2012. [data on file]
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

