Intratunical injection of human adipose tissue-derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of Peyronie's disease
- PMID: 23040209
- PMCID: PMC4029115
- DOI: 10.1016/j.eururo.2012.09.034
Intratunical injection of human adipose tissue-derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of Peyronie's disease
Erratum in
- Eur Urol. 2013 Jul;64(1):e21
Abstract
Background: Peyronie's disease (PD) is a connective tissue disorder of the tunica albuginea (TA). Currently, no gold standard has been developed for the treatment of the disease in its active phase.
Objective: To test the effects of a local injection of adipose tissue-derived stem cells (ADSCs) in the active phase of a rat model of PD on the subsequent development of fibrosis and elastosis of the TA and underlying erectile tissue.
Design, setting, and participants: A total of 27 male 12-wk-old Sprague-Dawley rats were divided in three equal groups and underwent injection of vehicle (sham), 0.5-μg [corrected] transforming growth factor (TGF)-β1 in a 50-μl vehicle in either a PD or a PD plus ADSC group in the dorsal aspect of the TA.
Intervention: The sham and PD groups were treated 1 d after TGF-β1 injection with intralesional treatment of vehicle, and the PD plus ADSC group received 1 million human-labeled ADSCs in the 50-μl vehicle. Five weeks after treatment, six rats per group underwent erectile function measurement. Following euthanasia, penises were harvested for histology and Western blot.
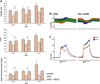
Outcome measurements and statistical analysis: The ratio of intracavernous pressure to mean arterial pressure (ICP/MAP) upon cavernous nerve stimulation, elastin, and collagen III protein expression and histomorphometric analysis of the penis. Statistical analysis was performed by analysis of variance followed by the Tukey-Kramer test for post hoc comparisons or the Mann-Whitney test when applicable.
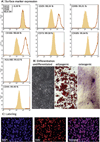
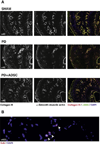
Results and limitations: Erectile function significantly improved after ADSC treatment (ICP/MAP 0.37 in PD vs 0.59 in PD plus ADSC at 5-V stimulation; p=0.03). PD animals developed areas of fibrosis and elastosis with a significant upregulation of collagen III and elastin protein expression. These fibrotic changes were prevented by ADSC treatment.
Conclusions: This study is the first to test stem cell therapy in an animal model of PD. Injection of ADSCs into the TA during the active phase of PD prevents the formation of fibrosis and elastosis in the TA and corpus cavernosum.
Copyright © 2012 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures




Comment in
-
Adipose-derived stem cells for the treatment of Peyronie's disease?Eur Urol. 2013 Mar;63(3):561-2. doi: 10.1016/j.eururo.2012.10.049. Epub 2012 Nov 5. Eur Urol. 2013. PMID: 23149148 Free PMC article. No abstract available.
Similar articles
-
Adipose tissue-derived stem cell therapy for prevention and treatment of erectile dysfunction in a rat model of Peyronie's disease.Andrology. 2014 Mar;2(2):244-51. doi: 10.1111/j.2047-2927.2013.00181.x. Andrology. 2014. PMID: 24574095
-
Intratunical Injection of Genetically Modified Adipose Tissue-Derived Stem Cells with Human Interferon α-2b for Treatment of Erectile Dysfunction in a Rat Model of Tunica Albugineal Fibrosis.J Sex Med. 2015 Jul;12(7):1533-44. doi: 10.1111/jsm.12916. Epub 2015 Jun 10. J Sex Med. 2015. PMID: 26062100 Free PMC article.
-
Intratunical Injection of Human Adipose Tissue-Derived Stem Cells Restores Collagen III/I Ratio in a Rat Model of Chronic Peyronie's Disease.Sex Med. 2019 Mar;7(1):94-103. doi: 10.1016/j.esxm.2018.09.003. Epub 2018 Nov 30. Sex Med. 2019. PMID: 30503767 Free PMC article.
-
[Biotherapies for erectile dysfunction and Peyronie's disease: Where are we now?].Prog Urol. 2020 Dec;30(16):1000-1013. doi: 10.1016/j.purol.2020.05.002. Epub 2020 Aug 18. Prog Urol. 2020. PMID: 32826194 French.
-
Peyronie's disease: new paradigm for the treatment of a unique cause of erectile dysfunction.Postgrad Med. 2020 Dec;132(sup4):4-8. doi: 10.1080/00325481.2020.1805865. Epub 2020 Nov 6. Postgrad Med. 2020. PMID: 33156731 Review.
Cited by
-
Prospects of stem cell treatment in benign urological diseases.Korean J Urol. 2015 Apr;56(4):257-65. doi: 10.4111/kju.2015.56.4.257. Epub 2015 Mar 30. Korean J Urol. 2015. PMID: 25874038 Free PMC article. Review.
-
Sexual dysfunction: The potential of stem cell therapy for Peyronie disease.Nat Rev Urol. 2013 Jan;10(1):8-9. doi: 10.1038/nrurol.2012.221. Epub 2012 Nov 20. Nat Rev Urol. 2013. PMID: 23165398 No abstract available.
-
Evolving therapies for Peyronie's disease: how can we work towards new drugs?Transl Androl Urol. 2020 Mar;9(Suppl 2):S284-S294. doi: 10.21037/tau.2019.08.09. Transl Androl Urol. 2020. PMID: 32257869 Free PMC article. Review.
-
Stem Cells and Regenerative Medicine: Myth or Reality of the 21th Century.Stem Cells Int. 2015;2015:734731. doi: 10.1155/2015/734731. Epub 2015 Aug 2. Stem Cells Int. 2015. PMID: 26300923 Free PMC article. Review.
-
Non-invasive treatment in the management of Peyronie's disease.Ther Adv Urol. 2019 Feb 11;11:1756287218823671. doi: 10.1177/1756287218823671. eCollection 2019 Jan-Dec. Ther Adv Urol. 2019. PMID: 30792820 Free PMC article. Review.
References
-
- Lin CS, Lin G, Wang Z, Maddah SA, Lue TF. Upregulation of monocyte chemoattractant protein 1 and effects of transforming growth factor-beta 1 in Peyronie’s disease. Biochem Biophys Res Commun. 2002;295:1014–1019. - PubMed
-
- Brock G, Hsu GL, Nunes L, von Heyden B, Lue TF. The anatomy of the tunica albuginea in the normal penis and Peyronie’s disease. J Urol. 1997;157:276–281. - PubMed
-
- Hatzimouratidis K, Eardley I, Giuliano F, et al. Guidelines on penile curvature. Eur Urol. 2012;62:543–552. - PubMed
-
- Ferretti L, Giuliani M, Bessède T, et al. Tissue engineering for penile surgery: comparative study of noncellular and cell-seeded synthetic grafts for tunica albuginea replacement. J Sex Med. 2012;9:625–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

