Neuropeptide transmission in brain circuits
- PMID: 23040809
- PMCID: PMC3918222
- DOI: 10.1016/j.neuron.2012.09.014
Neuropeptide transmission in brain circuits
Abstract
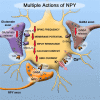
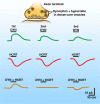
Neuropeptides are found in many mammalian CNS neurons where they play key roles in modulating neuronal activity. In contrast to amino acid transmitter release at the synapse, neuropeptide release is not restricted to the synaptic specialization, and after release, a neuropeptide may diffuse some distance to exert its action through a G protein-coupled receptor. Some neuropeptides such as hypocretin/orexin are synthesized only in single regions of the brain, and the neurons releasing these peptides probably have similar functional roles. Other peptides such as neuropeptide Y (NPY) are synthesized throughout the brain, and neurons that synthesize the peptide in one region have no anatomical or functional connection with NPY neurons in other brain regions. Here, I review converging data revealing a complex interaction between slow-acting neuromodulator peptides and fast-acting amino acid transmitters in the control of energy homeostasis, drug addiction, mood and motivation, sleep-wake states, and neuroendocrine regulation.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures







References
-
- Acsády L, Arabadzisz D, Freund TF. Correlated morphological and neurochemical features identify different subsets of vasoactive intestinal polypeptide-immunoreactive interneurons in rat hippocampus. Neuroscience. 1996;73:299–315. - PubMed
-
- Antonucci F, Alpár A, Kacza J, Caleo M, Verderio C, Giani A, Martens H, Chaudhry FA, Allegra M, Grosche J, Michalski D, Erck C, Hoffmann A, Harkany T, Matteoli M, Härtig W. Cracking down on inhibition: selective removal of GABAergic interneurons from hippocampal networks. J Neurosci. 2012;32:1989–2001. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

