Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior
- PMID: 23040810
- PMCID: PMC3466476
- DOI: 10.1016/j.neuron.2012.08.036
Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior
Abstract
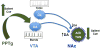
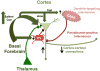
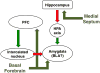
Acetylcholine in the brain alters neuronal excitability, influences synaptic transmission, induces synaptic plasticity, and coordinates firing of groups of neurons. As a result, it changes the state of neuronal networks throughout the brain and modifies their response to internal and external inputs: the classical role of a neuromodulator. Here, we identify actions of cholinergic signaling on cellular and synaptic properties of neurons in several brain areas and discuss consequences of this signaling on behaviors related to drug abuse, attention, food intake, and affect. The diverse effects of acetylcholine depend on site of release, receptor subtypes, and target neuronal population; however, a common theme is that acetylcholine potentiates behaviors that are adaptive to environmental stimuli and decreases responses to ongoing stimuli that do not require immediate action. The ability of acetylcholine to coordinate the response of neuronal networks in many brain areas makes cholinergic modulation an essential mechanism underlying complex behaviors.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Alkondon M, Pereira EF, Albuquerque EX. alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. - PubMed
-
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283:1396–1411. - PubMed
-
- Angelucci F, Brene S, Mathe AA. BDNF in schizophrenia, depression and corresponding animal models. Molecular psychiatry. 2005;10:345–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

