Neuromodulation of brain states
- PMID: 23040816
- PMCID: PMC3579548
- DOI: 10.1016/j.neuron.2012.09.012
Neuromodulation of brain states
Abstract
Switches between different behavioral states of the animal are associated with prominent changes in global brain activity, between sleep and wakefulness or from inattentive to vigilant states. What mechanisms control brain states, and what are the functions of the different states? Here we summarize current understanding of the key neural circuits involved in regulating brain states, with a particular emphasis on the subcortical neuromodulatory systems. At the functional level, arousal and attention can greatly enhance sensory processing, whereas sleep and quiet wakefulness may facilitate learning and memory. Several new techniques developed over the past decade promise great advances in our understanding of the neural control and function of different brain states.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

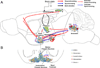
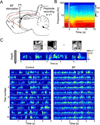



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

