Early tolerability and safety of fingolimod in clinical practice
- PMID: 23040960
- PMCID: PMC3970706
- DOI: 10.1016/j.jns.2012.09.009
Early tolerability and safety of fingolimod in clinical practice
Abstract
Background: Fingolimod is approved by the U.S. Food and Drug Administration to reduce relapses and disability progression in relapsing forms of MS. Several screening studies and a first-dose observation (FDO) period are recommended due to adverse effects observed in clinical trials.
Objective: The objective of this study is to describe the early experience with fingolimod, including startup, tolerability and safety in a large academic multiple sclerosis (MS) center.
Methods: Patients prescribed fingolimod from September 2010 to July 2011 were identified through electronic medical records. Demographics, MS disease history, pre-treatment screening studies, FDO experience during shared medical visits and three month follow-up data were analyzed.
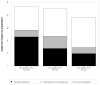
Results: Three hundred ninety-one patients were prescribed fingolimod of whom 317 started the medication and were included in the analysis. Fingolimod was most frequently used in relapsing remitting MS (n=256, 80.8%) and was prescribed as a first-line agent in 11 cases (3.5%). FDO was uneventful in 308 patients (96.8%). Adverse events during FDO were self limited and included symptomatic bradycardia (n=3), chest tightness (n=2) and hypertension (n=1). Fingolimod was discontinued in 30 patients (9.5%) at three months. Adverse effects leading to discontinuation by more than one patient included headache (n=4), macular edema (n=3), nausea (n=3) and hypertension (n=2).
Conclusions: Fingolimod was well tolerated during FDO and adverse events were self limited. The shared medical visit is an appropriate setting for FDO. Adverse effects were similar to those described in clinical trials but the discontinuation rate was higher.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Real-world use of fingolimod in patients with relapsing remitting multiple sclerosis: a retrospective study using the national multiple sclerosis registry in Kuwait.CNS Drugs. 2014 Sep;28(9):817-24. doi: 10.1007/s40263-014-0185-z. CNS Drugs. 2014. PMID: 25011422
-
A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis.N Engl J Med. 2010 Feb 4;362(5):387-401. doi: 10.1056/NEJMoa0909494. Epub 2010 Jan 20. N Engl J Med. 2010. PMID: 20089952 Clinical Trial.
-
Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial.Lancet Neurol. 2014 Jun;13(6):545-56. doi: 10.1016/S1474-4422(14)70049-3. Epub 2014 Mar 28. Lancet Neurol. 2014. PMID: 24685276 Clinical Trial.
-
Fingolimod: a review of its use in the management of relapsing-remitting multiple sclerosis.CNS Drugs. 2011 Aug;25(8):673-98. doi: 10.2165/11207350-000000000-00000. CNS Drugs. 2011. PMID: 21790210 Review.
-
Fingolimod-associated macular edema: incidence, detection, and management.Neurology. 2012 Feb 28;78(9):672-80. doi: 10.1212/WNL.0b013e318248deea. Neurology. 2012. PMID: 22371414 Review.
Cited by
-
Fingolimod in multiple sclerosis: profile of use in habitual practice.Eur J Hosp Pharm. 2020 Nov;27(6):346-349. doi: 10.1136/ejhpharm-2018-001840. Epub 2019 Mar 18. Eur J Hosp Pharm. 2020. PMID: 33097618 Free PMC article.
-
Sphingosine 1-Phosphate Receptor Modulators for the Treatment of Multiple Sclerosis.Neurotherapeutics. 2017 Oct;14(4):859-873. doi: 10.1007/s13311-017-0565-4. Neurotherapeutics. 2017. PMID: 28812220 Free PMC article. Review.
-
Fingolimod Associated Bilateral Cystoid Macular Edema-Wait and See?Int J Mol Sci. 2016 Dec 14;17(12):2106. doi: 10.3390/ijms17122106. Int J Mol Sci. 2016. PMID: 27983657 Free PMC article.
-
Real-world adherence to, and persistence with, once- and twice-daily oral disease-modifying drugs in patients with multiple sclerosis: a systematic review and meta-analysis.BMC Neurol. 2020 Jul 14;20(1):281. doi: 10.1186/s12883-020-01830-0. BMC Neurol. 2020. PMID: 32664928 Free PMC article.
-
Fingolimod: therapeutic mechanisms and ocular adverse effects.Eye (Lond). 2017 Feb;31(2):232-240. doi: 10.1038/eye.2016.258. Epub 2016 Nov 25. Eye (Lond). 2017. PMID: 27886183 Free PMC article. Review.
References
-
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–7. - PubMed
-
- Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. - PubMed
-
- Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L TRANSFORMS Study Group. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–15. - PubMed
-
- NDA 02257 FDA Approved Labeling Text for Gilenya (fingolimod) capsules. 2010 Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022527s000lbl.pdf.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources