The retinotopic organization of striate cortex is well predicted by surface topology
- PMID: 23041195
- PMCID: PMC3494819
- DOI: 10.1016/j.cub.2012.09.014
The retinotopic organization of striate cortex is well predicted by surface topology
Erratum in
- Curr Biol. 2012 Dec 4;22(23):2284
Abstract
In 1918, Gordon Holmes combined observations of visual-field scotomas across brain-lesioned soldiers to produce a schematic map of the projection of the visual field upon the striate cortex. One limit to the precision of his result, and the mapping of anatomy to retinotopy generally, is the substantial individual variation in the size, volumetric position, and cortical magnification of area V1. When viewed within the context of the curvature of the cortical surface, however, the boundaries of striate cortex fall at a consistent location across individuals. We asked whether the surface topology of the human brain can be used to accurately predict the internal, retinotopic function of striate cortex as well. We used fMRI to measure polar angle and eccentricity in 25 participants and combined their maps within a left-right, transform-symmetric representation of the cortical surface. These data were then fit using a deterministic, algebraic model of visual-field representation. We found that an anatomical image alone can be used to predict the retinotopic organization of striate cortex for an individual with accuracy equivalent to 10-25 min of functional mapping. This indicates tight developmental linkage of structure and function within a primary, sensory cortical area.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
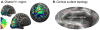

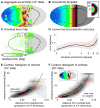
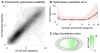
Comment in
-
Brain mapping: the (un)folding of striate cortex.Curr Biol. 2012 Dec 18;22(24):R1051-3. doi: 10.1016/j.cub.2012.11.003. Curr Biol. 2012. PMID: 23257192
References
-
- Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA. Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J Vis. 2003;3(10):586–598. - PubMed
-
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. Brodmann’s areas 17 and 18 brought into stereotaxic space-where and how variable? NeuroImage. 2000;11(1):66–84. - PubMed
-
- Qiu A, Rosenau BJ, Greenberg AS, Hurdal MK, Barta P, Yantis S, Miller MI. Estimating linear cortical magnification in human primary visual cortex via dynamic programming. NeuroImage. 2006;31(1):125–138. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

