Gene density, transcription, and insulators contribute to the partition of the Drosophila genome into physical domains
- PMID: 23041285
- PMCID: PMC3496039
- DOI: 10.1016/j.molcel.2012.08.031
Gene density, transcription, and insulators contribute to the partition of the Drosophila genome into physical domains
Abstract
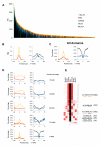
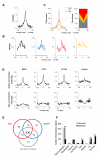
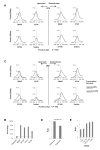
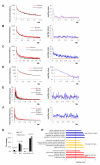
The mechanisms responsible for the establishment of physical domains in metazoan chromosomes are poorly understood. Here we find that physical domains in Drosophila chromosomes are demarcated at regions of active transcription and high gene density that are enriched for transcription factors and specific combinations of insulator proteins. Physical domains contain different types of chromatin defined by the presence of specific proteins and epigenetic marks, with active chromatin preferentially located at the borders and silenced chromatin in the interior. Domain boundaries participate in long-range interactions that may contribute to the clustering of regions of active or silenced chromatin in the nucleus. Analysis of transgenes suggests that chromatin is more accessible and permissive to transcription at the borders than inside domains, independent of the presence of active or silencing histone modifications. These results suggest that the higher-order physical organization of chromatin may impose an additional level of regulation over classical epigenetic marks.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Order from chaos in the nucleus.Mol Cell. 2012 Nov 9;48(3):327-8. doi: 10.1016/j.molcel.2012.10.021. Mol Cell. 2012. PMID: 23141201 Free PMC article.
References
-
- Bantignies F, Roure V, Comet I, Leblanc B, Schuettengruber B, Bonnet J, Tixier V, Mas A, Cavalli G. Polycomb-dependent regulatory contacts between distant Hox loci in Drosophila. Cell. 2011;144:214–226. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

