Developmental plasticity is bound by pluripotency and the Fgf and Wnt signaling pathways
- PMID: 23041313
- PMCID: PMC3607220
- DOI: 10.1016/j.celrep.2012.08.029
Developmental plasticity is bound by pluripotency and the Fgf and Wnt signaling pathways
Abstract
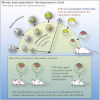
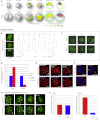
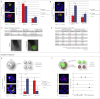
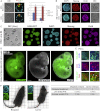
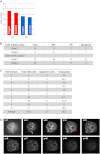
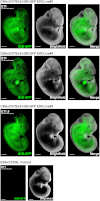
Plasticity is a well-known feature of mammalian development, and yet very little is known about its underlying mechanism. Here, we establish a model system to examine the extent and limitations of developmental plasticity in living mouse embryos. We show that halved embryos follow the same strict clock of developmental transitions as intact embryos, but their potential is not equal. We have determined that unless a minimum of four pluripotent cells is established before implantation, development will arrest. This failure can be rescued by modulating Fgf and Wnt signaling to enhance pluripotent cell number, allowing the generation of monozygotic twins, which is an otherwise rare phenomenon. Knowledge of the minimum pluripotent-cell number required for development to birth, as well as the different potentials of blastomeres, allowed us to establish a protocol for splitting an embryo into one part that develops to adulthood and another that provides embryonic stem cells for that individual.
Copyright © 2012 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Chazaud C., Yamanaka Y., Pawson T., Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev. Cell. 2006;10:615–624. - PubMed
Supplemental References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

