The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice
- PMID: 23041323
- PMCID: PMC6054127
- DOI: 10.1053/j.gastro.2012.09.055
The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice
Abstract
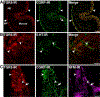
Background & aims: Abnormal delivery of bile acids (BAs) to the colon as a result of disease or therapy causes constipation or diarrhea by unknown mechanisms. The G protein-coupled BA receptor TGR5 (or GPBAR1) is expressed by enteric neurons and endocrine cells, which regulate motility and secretion.
Methods: We analyzed gastrointestinal and colon transit, as well as defecation frequency and water content, in wild-type, knockout, and transgenic mice (trg5-wt, tgr5-ko, and tgr5-tg, respectively). We analyzed colon tissues for contractility, peristalsis, and transmitter release.
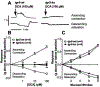
Results: Deoxycholic acid inhibited contractility of colonic longitudinal muscle from tgr5-wt but not tgr5-ko mice. Application of deoxycholic acid, lithocholic acid, or oleanolic acid (a selective agonist of TGR5) to the mucosa of tgr5-wt mice caused oral contraction and caudal relaxation, indicating peristalsis. BAs stimulated release of the peristaltic transmitters 5-hydroxytryptamine and calcitonin gene-related peptide; antagonists of these transmitters suppressed BA-induced peristalsis, consistent with localization of TGR5 to enterochromaffin cells and intrinsic primary afferent neurons. tgr5-ko mice did not undergo peristalsis or transmitter release in response to BAs. Mechanically induced peristalsis and transmitter release were not affected by deletion of tgr5. Whole-gut transit was 1.4-fold slower in tgr5-ko than tgr5-wt or tgr5-tg mice, whereas colonic transit was 2.2-fold faster in tgr5-tg mice. Defecation frequency was reduced 2.6-fold in tgr5-ko and increased 1.4-fold in tgr5-tg mice compared with tgr5-wt mice. Water content in stool was lower (37%) in tgr5-ko than tgr5-tg (58%) or tgr5-wt mice (62%).
Conclusions: The receptor TGR5 mediates the effects of BAs on colonic motility, and deficiency of TGR5 causes constipation in mice. These findings might mediate the long-known laxative properties of BAs, and TGR5 might be a therapeutic target for digestive diseases.
Copyright © 2013 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures


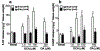
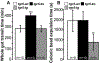
References
-
- Bajor A, Gillberg PG, Abrahamsson H. Bile acids: short and long term effects in the intestine. Scandinavian journal of gastroenterology 2010;45:645–664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

