The onco-embryonic antigen ROR1 is expressed by a variety of human cancers
- PMID: 23041612
- PMCID: PMC3509760
- DOI: 10.1016/j.ajpath.2012.08.024
The onco-embryonic antigen ROR1 is expressed by a variety of human cancers
Abstract
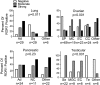
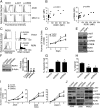
ROR1 is an orphan-receptor tyrosine-kinase-like surface antigen that is expressed by many tissues during embryogenesis, some B-cell malignancies, and various cancer cell lines but not by virtually all normal adult tissues. Here, we report that large proportions of many different human cancers also express ROR1, particularly those cancers that have high-grade histology. Primary cancers that expressed ROR1 more commonly expressed high levels of phosphorylated AKT (p-AKT) and phosphorylated cAMP response element binding-factor (p-CREB) than similar cancers that lacked expression of ROR1. Induced expression of ROR1 could enhance basal p-AKT and p-CREB levels and could promote the growth of a cancer cell line, MEC1. Conversely, silencing ROR1 resulted in lower levels of p-AKT and p-CREB, which was associated with impaired tumor cell growth. In summary, this study found that many different human cancers express ROR1 and that ROR1 may play a functional role in promoting tumor cell growth, suggesting that this orphan-receptor tyrosine-kinase-like protein may be a potential target for therapy directed against a variety of human cancers.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Barna G., Mihalik R., Timar B., Tombol J., Csende Z., Sebestyen A., Bodor C., Csernus B., Reiniger L., Petak I., Matolcsy A. ROR1 expression is not a unique marker of CLL. Hematol Oncol. 2011;29:17–21. - PubMed
-
- Baskar S., Kwong K.Y., Hofer T., Levy J.M., Kennedy M.G., Lee E., Staudt L.M., Wilson W.H., Wiestner A., Rader C. Unique cell surface expression of receptor tyrosine kinase ROR1 in human B-cell chronic lymphocytic leukemia. Clin Cancer Res. 2008;14:396–404. - PubMed
-
- Daneshmanesh A.H., Mikaelsson E., Jeddi-Tehrani M., Bayat A.A., Ghods R., Ostadkarampour M., Akhondi M., Lagercrantz S., Larsson C., Osterborg A., Shokri F., Mellstedt H., Rabbani H. Ror1, a cell surface receptor tyrosine kinase is expressed in chronic lymphocytic leukemia and may serve as a putative target for therapy. Int J Cancer. 2008;123:1190–1195. - PubMed
-
- Fukuda T., Chen L., Endo T., Tang L., Lu D., Castro J.E., Widhopf G.F., II, Rassenti L.Z., Cantwell M.J., Prussak C.E., Carson D.A., Kipps T.J. Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc Natl Acad Sci U S A. 2008;105:3047–3052. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

