AFG3L2 supports mitochondrial protein synthesis and Purkinje cell survival
- PMID: 23041622
- PMCID: PMC3484458
- DOI: 10.1172/JCI64604
AFG3L2 supports mitochondrial protein synthesis and Purkinje cell survival
Abstract
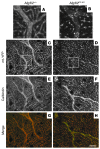
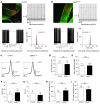
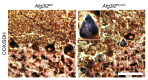
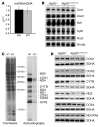
Mutations in the AFG3L2 gene have been linked to spinocerebellar ataxia type 28 and spastic ataxia-neuropathy syndrome in humans; however, the pathogenic mechanism is still unclear. AFG3L2 encodes a subunit of the mitochondrial m-AAA protease, previously implicated in quality control of misfolded inner mitochondrial membrane proteins and in regulatory functions via processing of specific substrates. Here, we used a conditional Afg3l2 mouse model that allows restricted deletion of the gene in Purkinje cells (PCs) to shed light on the pathogenic cascade in the neurons mainly affected in the human diseases. We demonstrate a cell-autonomous requirement of AFG3L2 for survival of PCs. Examination of PCs prior to neurodegeneration revealed fragmentation and altered distribution of mitochondria in the dendritic tree, indicating that abnormal mitochondrial dynamics is an early event in the pathogenic process. Moreover, PCs displayed features pointing to defects in mitochondrially encoded respiratory chain subunits at early stages. To unravel the underlying mechanism, we examined a constitutive knockout of Afg3l2, which revealed a decreased rate of mitochondrial protein synthesis associated with impaired mitochondrial ribosome assembly. We therefore propose that defective mitochondrial protein synthesis, leading to early-onset fragmentation of the mitochondrial network, is a central causative factor in AFG3L2-related neurodegeneration.
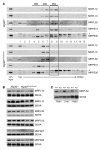
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

