C1 catecholamine neurons form local circuit synaptic connections within the rostroventrolateral medulla of rat
- PMID: 23041757
- PMCID: PMC3538374
- DOI: 10.1016/j.neuroscience.2012.09.049
C1 catecholamine neurons form local circuit synaptic connections within the rostroventrolateral medulla of rat
Abstract
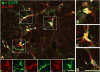
C1 catecholamine neurons reside within the rostroventrolateral medulla (RVLM), an area that plays an integral role in blood pressure regulation through reticulospinal projections to sympathetic preganglionic neurons in the thoracic spinal cord. In a previous investigation we mapped the efferent projections of C1 neurons, documenting supraspinal projections to cell groups in the preautonomic network that contribute to the control of cardiovascular function. Light microscopic study also revealed putative local circuit connections within RVLM. In this investigation we tested the hypothesis that RVLM C1 neurons elaborate a local circuit synaptic network that permits communication between C1 neurons giving rise to supraspinal and reticulospinal projections. A replication defective lentivirus vector that expresses enhanced green fluorescent protein (EGFP) under the control of a synthetic dopamine beta hydroxylase (DβH) promoter was used to label C1 neurons and their processes. Confocal fluorescence microscopy demonstrated thin varicose axons immunopositive for EGFP and tyrosine hydroxylase that formed close appositions to C1 somata and dendrites throughout the rostrocaudal extent of the C1 area. Dual-labeled electron microscopic analysis revealed axosomatic, axodendritic and axospinous synaptic contacts with C1 and non-C1 neurons with a distribution recapitulating that observed in the light microscopic analysis. Labeled boutons were large, contained light axoplasm, lucent spherical vesicles, and formed asymmetric synaptic contacts. Collectively these data demonstrate that C1 neurons form a synaptic network within the C1 area that may function to coordinate activity among projection-specific subpopulations of neurons. The data also suggest that the boundaries of RVLM should be defined on the basis of function criteria rather than the C1 phenotype of neurons.
Copyright © 2012 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures




References
-
- Aicher SA, Kraus JA, Sharma S, Patel A, Milner TA. Selective distribution of mu-opioid receptors in C1 adrenergic neurons and their afferents. Journal of Comparative Neurology. 2001;433:23–33. - PubMed
-
- Barman SM, Gebber GL. Lateral tegmental field neurons of cat medulla: A source of basal activity of ventrolateral medullospinal sympathoexcitatory neurons. Journal of Neurophysiology. 1987;57:1410–1424. - PubMed
-
- Blessing WW, Hedger SC, Joh TH, Willoughby JO. Neurons in the area postrema are the only catecholamine-synthesizing cells in the medulla or pons with projections to the rostral ventrolateral medulla (C1-area) in the rabbit. Brain Research. 1987;419:336–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

