LCR 5' hypersensitive site specificity for globin gene activation within the active chromatin hub
- PMID: 23042246
- PMCID: PMC3526258
- DOI: 10.1093/nar/gks900
LCR 5' hypersensitive site specificity for globin gene activation within the active chromatin hub
Abstract
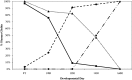
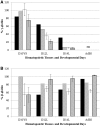
The DNaseI hypersensitive sites (HSs) of the human β-globin locus control region (LCR) may function as part of an LCR holocomplex within a larger active chromatin hub (ACH). Differential activation of the globin genes during development may be controlled in part by preferential interaction of each gene with specific individual HSs during globin gene switching, a change in conformation of the LCR holocomplex, or both. To distinguish between these possibilities, human β-globin locus yeast artificial chromosome (β-YAC) lines were produced in which the ε-globin gene was replaced with a second marked β-globin gene (β(m)), coupled to an intact LCR, a 5'HS3 complete deletion (5'ΔHS3) or a 5'HS3 core deletion (5'ΔHS3c). The 5'ΔHS3c mice expressed β(m)-globin throughout development; γ-globin was co-expressed in the embryonic yolk sac, but not in the fetal liver; and wild-type β-globin was co-expressed in adult mice. Although the 5'HS3 core was not required for β(m)-globin expression, previous work showed that the 5'HS3 core is necessary for ε-globin expression during embryonic erythropoiesis. A similar phenotype was observed in 5'HS complete deletion mice, except β(m)-globin expression was higher during primitive erythropoiesis and γ-globin expression continued into fetal definitive erythropoiesis. These data support a site specificity model of LCR HS-globin gene interaction.
Figures







Similar articles
-
Synergistic and additive properties of the beta-globin locus control region (LCR) revealed by 5'HS3 deletion mutations: implication for LCR chromatin architecture.Mol Cell Biol. 2005 Aug;25(16):7033-41. doi: 10.1128/MCB.25.16.7033-7041.2005. Mol Cell Biol. 2005. PMID: 16055715 Free PMC article.
-
Effect of deletion of 5'HS3 or 5'HS2 of the human beta-globin locus control region on the developmental regulation of globin gene expression in beta-globin locus yeast artificial chromosome transgenic mice.Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6605-9. doi: 10.1073/pnas.93.13.6605. Proc Natl Acad Sci U S A. 1996. PMID: 8692864 Free PMC article.
-
The human beta-globin locus control region confers an early embryonic erythroid-specific expression pattern to a basic promoter driving the bacterial lacZ gene.Development. 1996 Dec;122(12):3991-9. doi: 10.1242/dev.122.12.3991. Development. 1996. PMID: 9012519
-
beta-YAC transgenic mice for studying LCR function.Ann N Y Acad Sci. 1998 Jun 30;850:28-37. doi: 10.1111/j.1749-6632.1998.tb10459.x. Ann N Y Acad Sci. 1998. PMID: 9668524 Review.
-
Joining the loops: beta-globin gene regulation.IUBMB Life. 2008 Dec;60(12):824-33. doi: 10.1002/iub.129. IUBMB Life. 2008. PMID: 18767169 Review.
Cited by
-
Improved Titer and Gene Transfer by Lentiviral Vectors Using Novel, Small β-Globin Locus Control Region Elements.Mol Ther. 2020 Jan 8;28(1):328-340. doi: 10.1016/j.ymthe.2019.09.020. Epub 2019 Sep 28. Mol Ther. 2020. PMID: 31628051 Free PMC article.
-
Heterozygosity for deletion of hypersensitive site 3 in the human locus control region has an unexpected minor effect on red cell phenotype.J Hum Genet. 2014 Oct;59(10):585-7. doi: 10.1038/jhg.2014.76. Epub 2014 Sep 4. J Hum Genet. 2014. PMID: 25186056
-
Original Research: Generation of non-deletional hereditary persistence of fetal hemoglobin β-globin locus yeast artificial chromosome transgenic mouse models: -175 Black HPFH and -195 Brazilian HPFH.Exp Biol Med (Maywood). 2016 Apr;241(7):697-705. doi: 10.1177/1535370216636724. Epub 2016 Mar 4. Exp Biol Med (Maywood). 2016. PMID: 26946532 Free PMC article.
-
Creating New β-Globin-Expressing Lentiviral Vectors by High-Resolution Mapping of Locus Control Region Enhancer Sequences.Mol Ther Methods Clin Dev. 2020 Apr 18;17:999-1013. doi: 10.1016/j.omtm.2020.04.006. eCollection 2020 Jun 12. Mol Ther Methods Clin Dev. 2020. PMID: 32426415 Free PMC article.
References
-
- Stamatoyannopoulos G, Grosveld F. Hemoglobin switching. In: Stamatoyannopoulos G, Majerus PW, Perlmutter RM, Varmus H, editors. The Molecular Basis of Blood Diseases. 3rd edn. Philadelphia, PA: W.B. Saunders; 2001. pp. 135–182.
-
- Harju S, McQueen KJ, Peterson KR. Chromatin structure and control of β-like globin gene switching. Exp. Biol. Med. 2002;227:683–700. - PubMed
-
- Noordermeer D, de Laat W. Joining the loops: β-globin gene regulation. IUBMB Life. 2008;60:824–833. - PubMed

