IRE1α cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2
- PMID: 23042294
- PMCID: PMC3742121
- DOI: 10.1126/science.1226191
IRE1α cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2
Abstract
The endoplasmic reticulum (ER) is the primary organelle for folding and maturation of secretory and transmembrane proteins. Inability to meet protein-folding demand leads to "ER stress," and activates IRE1α, an ER transmembrane kinase-endoribonuclease (RNase). IRE1α promotes adaptation through splicing Xbp1 mRNA or apoptosis through incompletely understood mechanisms. Here, we found that sustained IRE1α RNase activation caused rapid decay of select microRNAs (miRs -17, -34a, -96, and -125b) that normally repress translation of Caspase-2 mRNA, and thus sharply elevates protein levels of this initiator protease of the mitochondrial apoptotic pathway. In cell-free systems, recombinant IRE1α endonucleolytically cleaved microRNA precursors at sites distinct from DICER. Thus, IRE1α regulates translation of a proapoptotic protein through terminating microRNA biogenesis, and noncoding RNAs are part of the ER stress response.
Figures
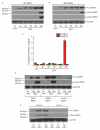

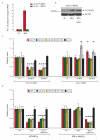

Comment in
-
IRE1, a double-edged sword in pre-miRNA slicing and cell death.Dev Cell. 2012 Nov 13;23(5):921-3. doi: 10.1016/j.devcel.2012.10.025. Dev Cell. 2012. PMID: 23153490 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK063720/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 CA136717/CA/NCI NIH HHS/United States
- R01CA154916/CA/NCI NIH HHS/United States
- R01 DK080955/DK/NIDDK NIH HHS/United States
- DP2 OD001925/OD/NIH HHS/United States
- GM080783/GM/NIGMS NIH HHS/United States
- R01 CA136577/CA/NCI NIH HHS/United States
- R01CA136577/CA/NCI NIH HHS/United States
- R01 CA140456/CA/NCI NIH HHS/United States
- R01 CA154916/CA/NCI NIH HHS/United States
- R01DK080955/DK/NIDDK NIH HHS/United States
- DK063720/DK/NIDDK NIH HHS/United States
- R01CA136717/CA/NCI NIH HHS/United States
- R01 GM080783/GM/NIGMS NIH HHS/United States
- DP2OD001925/OD/NIH HHS/United States
- R01CA140456/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

