Exercise alters mRNA expression of telomere-repeat binding factor 1 in skeletal muscle via p38 MAPK
- PMID: 23042912
- PMCID: PMC3544504
- DOI: 10.1152/japplphysiol.00200.2012
Exercise alters mRNA expression of telomere-repeat binding factor 1 in skeletal muscle via p38 MAPK
Abstract
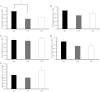
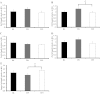
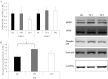
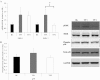
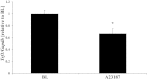
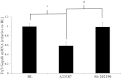
Telomeres protect chromosome ends and shorten with age in most tissues. Integral to the maintenance of telomeres is the protein complex shelterin. The gene expression regulation of shelterin proteins to physiological stressors is not understood in vivo. We have recently reported increased telomere-repeat binding factor 1 (TRF1) protein expression and longer telomere length in skeletal muscle of sedentary compared with chronically active mice. These provocative observations led us to examine the effects of acute physiological stress on shelterin expression in vivo in mice and to further define potential mechanisms associated with gene regulation of shelterin. Three groups of female C57Bl/6 mice were studied: one control group and two groups that underwent a 30-min treadmill running bout and were killed either immediately following or 1-h after the exercise. Following the exercise bout, mRNA expression of Trf1 was significantly reduced in the plantaris muscle, and this reduction was paralleled by significant increases in p38 MAPK phosphorylation. To determine if p38 mediated the decreases in Trf1 mRNA expression, C2C12 myotubes were treated with the calcium ionophore, A23187. In response to the A23187, Trf1 gene expression was significantly reduced, coupled with significant increases in p38 phosphorylation, similar to in vivo data. C2C12 myotubes pretreated with a p38 inhibitor (SB-202190) prevented the A23187-induced decrease in Trf1 mRNA expression, indicating a link between Trf1 gene expression and p38 MAPK activation. While it is too early to definitively report the effect of exercise on telomere biology in rodents or humans, these data provide important mechanistic insights into the paradoxical telomere shortening that occurs in skeletal muscle in response to chronic exercise in mice.
Figures

Similar articles
-
Acute exercise activates p38 MAPK and increases the expression of telomere-protective genes in cardiac muscle.Exp Physiol. 2017 Apr 1;102(4):397-410. doi: 10.1113/EP086189. Epub 2017 Mar 14. Exp Physiol. 2017. PMID: 28166612 Free PMC article.
-
Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway.J Biol Chem. 2005 May 20;280(20):19587-93. doi: 10.1074/jbc.M408862200. Epub 2005 Mar 14. J Biol Chem. 2005. PMID: 15767263
-
Coordinate regulation between expression levels of telomere-binding proteins and telomere length in breast carcinomas.Cancer Med. 2012 Oct;1(2):165-75. doi: 10.1002/cam4.14. Epub 2012 Jul 24. Cancer Med. 2012. PMID: 23342266 Free PMC article.
-
Post-translational modifications of TRF1 and TRF2 and their roles in telomere maintenance.Mech Ageing Dev. 2012 Jun;133(6):421-34. doi: 10.1016/j.mad.2012.05.002. Epub 2012 May 23. Mech Ageing Dev. 2012. PMID: 22634377 Review.
-
TRF1 and TRF2: pioneering targets in telomere-based cancer therapy.J Cancer Res Clin Oncol. 2024 Jul 16;150(7):353. doi: 10.1007/s00432-024-05867-3. J Cancer Res Clin Oncol. 2024. PMID: 39012375 Free PMC article. Review.
Cited by
-
Do telomeres adapt to physiological stress? Exploring the effect of exercise on telomere length and telomere-related proteins.Biomed Res Int. 2013;2013:601368. doi: 10.1155/2013/601368. Epub 2013 Dec 24. Biomed Res Int. 2013. PMID: 24455708 Free PMC article. Review.
-
Telomere Length Maintenance and Cardio-Metabolic Disease Prevention Through Exercise Training.Sports Med. 2016 Sep;46(9):1213-37. doi: 10.1007/s40279-016-0482-4. Sports Med. 2016. PMID: 26914269 Review.
-
Acute forced exercise increases Bdnf IV mRNA and reduces exploratory behavior in C57BL/6J mice.Genes Brain Behav. 2020 Jun;19(5):e12617. doi: 10.1111/gbb.12617. Epub 2019 Nov 5. Genes Brain Behav. 2020. PMID: 31621198 Free PMC article.
-
BRCA1 is a novel regulator of metabolic function in skeletal muscle.J Lipid Res. 2014 Apr;55(4):668-80. doi: 10.1194/jlr.M043851. Epub 2014 Feb 24. J Lipid Res. 2014. PMID: 24565757 Free PMC article.
-
Telomere attrition: metabolic regulation and signalling function?Biol Lett. 2019 Mar 29;15(3):20180885. doi: 10.1098/rsbl.2018.0885. Biol Lett. 2019. PMID: 30890069 Free PMC article.
References
-
- Aguennouz M, Vita GL, Messina S, Cama A, Lanzano N, Ciranni A, Rodolico C, Di Giorgio RM, Vita G. Telomere shortening is associated to TRF1 and PARP1 overexpression in Duchenne muscular dystrophy. Neurobiol Aging 32: 2190–2197, 2011 - PubMed
-
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 280: 19587–19593, 2005 - PubMed
-
- Allen DL, Leinwand LA. Intracellular calcium and myosin isoform transitions. Calcineurin and calcium-calmodulin kinase pathways regulate preferential activation of the IIa myosin heavy chain promoter. J Biol Chem 277: 45323–45330, 2002 - PubMed
-
- Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett 579: 859–862, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

