Safety, tolerability and pharmacokinetics of a human anti-interleukin-13 monoclonal antibody (CNTO 5825) in an ascending single-dose first-in-human study
- PMID: 23043368
- PMCID: PMC3635599
- DOI: 10.1111/j.1365-2125.2012.04477.x
Safety, tolerability and pharmacokinetics of a human anti-interleukin-13 monoclonal antibody (CNTO 5825) in an ascending single-dose first-in-human study
Abstract
Aims: To assess the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD) and immunogenicity of CNTO 5825 following single-dose intravenous (i.v.) and subcutaneous (s.c.) administration in healthy and healthy atopic subjects.
Methods: Sixty-four subjects received a single dose of placebo or CNTO 5825 (0.1, 0.3, 1.0, 3.0, or 10 mg kg(-1) i.v. in a dose-escalating manner, or 3.0 mg kg(-1) s.c. in healthy subjects; and 10 mg kg(-1) i.v. in healthy atopic subjects). Subjects were observed for 96 h postadministration and followed for 16 weeks. Safety and tolerability were monitored, and serum samples were collected to measure CNTO 5825 concentrations, antibodies to CNTO 5825 and PD biomarkers.
Results: Most adverse events were mild to moderate in severity and considered to be unrelated to CNTO 5825, with no dose-dependent trends seen. The two serious adverse events were considered to be unrelated to CNTO 5825. After i.v. administration, CNTO 5825 exhibited linear PK, with a terminal half-life of ∼22-32 days. After a single 3 mg kg(-1) s.c. dose in healthy subjects, CNTO 5825 was absorbed into the systemic circulation with a median time to maximum serum concentration (tmax) of 5.45 days and absolute bioavailability of ∼75%. The PK profile of CNTO 5825 at 10 mg kg(-1) was similar in both healthy and healthy atopic subjects. No antibodies to CNTO 5825 were detected through week 16. In the CNTO 5825-treated healthy atopic subjects, there was a significant reduction in serum IgE and C-C motif chemokine ligand 17 (P = 0.028 and 0.068 vs. placebo, respectively).
Conclusions: CNTO 5825 was well tolerated, had an acceptable safety profile, exhibited linear PK characteristics, and no detected antibodies to CNTO 5825.
Trial registration: ClinicalTrials.gov NCT01081691.
© 2012 Janssen Research & Development, LLC. British Journal of Clinical Pharmacology © 2012 The British Pharmacological Society.
Figures

 , 0.1 mg kg–1 i.v.;
, 0.1 mg kg–1 i.v.;  , 0.3 mg kg–1 i.v.;
, 0.3 mg kg–1 i.v.;  , 1.0 mg kg–1 i.v.;
, 1.0 mg kg–1 i.v.;  , 3.0 mg kg–1 i.v.;
, 3.0 mg kg–1 i.v.;  , 10 mg kg–1 i.v.;
, 10 mg kg–1 i.v.;  , 10 mg kg–1 i.v., atopic subjects;
, 10 mg kg–1 i.v., atopic subjects;  , 3.0 mg kg–1 s.c.
, 3.0 mg kg–1 s.c.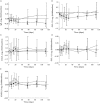
 , placebo;
, placebo;  , CNTO 5825
, CNTO 5825Similar articles
-
Pharmacokinetics, pharmacodynamics and safety of a human anti-IL-6 monoclonal antibody (sirukumab) in healthy subjects in a first-in-human study.Br J Clin Pharmacol. 2011 Aug;72(2):270-81. doi: 10.1111/j.1365-2125.2011.03964.x. Br J Clin Pharmacol. 2011. PMID: 21392075 Free PMC article. Clinical Trial.
-
The first-in-human study of CNTO 7160, an anti-interleukin-33 receptor monoclonal antibody, in healthy subjects and patients with asthma or atopic dermatitis.Br J Clin Pharmacol. 2020 Dec;86(12):2507-2518. doi: 10.1111/bcp.14361. Epub 2020 Jun 14. Br J Clin Pharmacol. 2020. PMID: 32415720 Free PMC article. Clinical Trial.
-
A phase I, single and fractionated, ascending-dose study evaluating the safety, pharmacokinetics, pharmacodynamics, and immunogenicity of an erythropoietin mimetic antibody fusion protein (CNTO 528) in healthy male subjects.J Clin Pharmacol. 2008 Oct;48(10):1197-207. doi: 10.1177/0091270008322907. J Clin Pharmacol. 2008. PMID: 18812609 Clinical Trial.
-
Pharmacokinetics, Pharmacodynamics, Safety, and Tolerability of Stapokibart in Healthy Volunteers and Adult Subjects with Atopic Dermatitis.Adv Ther. 2024 Jul;41(7):2953-2965. doi: 10.1007/s12325-024-02887-w. Epub 2024 Jun 4. Adv Ther. 2024. PMID: 38833140 Clinical Trial.
-
A Double-Blind, Phase I, Single Ascending Dose Study to Assess the Safety, Pharmacokinetics, and Pharmacodynamics of BOS161721 in Healthy Subjects.Clin Transl Sci. 2020 Mar;13(2):337-344. doi: 10.1111/cts.12715. Epub 2019 Nov 29. Clin Transl Sci. 2020. PMID: 31664766 Free PMC article. Clinical Trial.
Cited by
-
A Mechanistic Site-Of-Action Model: A Tool for Informing Right Target, Right Compound, And Right Dose for Therapeutic Antagonistic Antibody Programs.Front Bioinform. 2021 Sep 3;1:731340. doi: 10.3389/fbinf.2021.731340. eCollection 2021. Front Bioinform. 2021. PMID: 36303796 Free PMC article.
-
Mechanistic modeling of a human IgG4 monoclonal antibody (tralokinumab) Fab-arm exchange with endogenous IgG4 in healthy volunteers.CPT Pharmacometrics Syst Pharmacol. 2022 Apr;11(4):438-446. doi: 10.1002/psp4.12738. Epub 2022 Jan 12. CPT Pharmacometrics Syst Pharmacol. 2022. PMID: 35023315 Free PMC article.
-
Simple Approach to Accurately Predict Pharmacokinetics of Therapeutic Monoclonal Antibodies after Subcutaneous Injection in Humans.Clin Pharmacokinet. 2021 Jan;60(1):111-120. doi: 10.1007/s40262-020-00917-8. Clin Pharmacokinet. 2021. PMID: 32779124
-
Estimation of Clearance and Bioavailability of Therapeutic Monoclonal Antibodies from Only Subcutaneous Injection Data in Humans Based on Comprehensive Analysis of Clinical Data.Clin Pharmacokinet. 2021 Oct;60(10):1325-1334. doi: 10.1007/s40262-021-01023-z. Epub 2021 May 6. Clin Pharmacokinet. 2021. PMID: 33954956 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

