Local structure and global patterning of Cu2+ binding in fibrillar amyloid-β [Aβ(1-40)] protein
- PMID: 23043377
- PMCID: PMC3722434
- DOI: 10.1021/ja306946q
Local structure and global patterning of Cu2+ binding in fibrillar amyloid-β [Aβ(1-40)] protein
Abstract
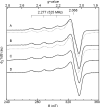
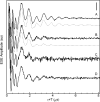
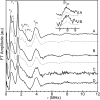
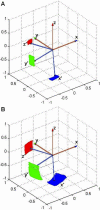
The amyloid-β (Aβ) protein forms fibrils and higher-order plaque aggegrates in Alzheimer's disease (AD) brain. The copper ion, Cu(2+), is found at high concentrations in plaques, but its role in AD etiology is unclear. We use high-resolution pulsed electron paramagnetic resonance spectroscopy to characterize the coordination structure of Cu(2+) in the fibrillar form of full-length Aβ(1-40). The results reveal a bis-cis-histidine (His) equatorial Cu(2+) coordination geometry and participation of all three N-terminal His residues in Cu(2+) binding. A model is proposed in which Cu(2+)-His6/His13 and Cu(2+)-His6/His14 sites alternate along the fibril axis on opposite sides of the β-sheet fibril structure. The local intra-β-strand coordination structure is not conducive to Cu(2+)/Cu(+) redox-linked coordination changes, and the global arrangement of Cu sites precludes facile multielectron and bridged-metal site reactivity. This indicates that the fibrillar form of Aβ suppresses Cu redox cycling and reactive oxygen species production. The configuration suggests application of Cu(2+)-Aβ fibrils as an amyloid architecture for switchable electron charge/spin coupling and redox reactivity.
Figures


Similar articles
-
Substantial contribution of the two imidazole rings of the His13-His14 dyad to Cu(II) binding in amyloid-β(1-16) at physiological pH and its significance.J Phys Chem A. 2011 Sep 1;115(34):9590-602. doi: 10.1021/jp200379m. Epub 2011 Apr 14. J Phys Chem A. 2011. PMID: 21491887 Free PMC article.
-
Using N-Terminal Coordination of Cu(II) and Ni(II) to Isolate the Coordination Environment of Cu(I) and Cu(II) Bound to His13 and His14 in Amyloid-β(4-16).Inorg Chem. 2019 Nov 18;58(22):15138-15154. doi: 10.1021/acs.inorgchem.9b01940. Epub 2019 Oct 28. Inorg Chem. 2019. PMID: 31657204
-
The amyloid-beta peptide of Alzheimer's disease binds Cu(I) in a linear bis-his coordination environment: insight into a possible neuroprotective mechanism for the amyloid-beta peptide.J Am Chem Soc. 2008 Dec 31;130(52):17826-35. doi: 10.1021/ja805940m. J Am Chem Soc. 2008. PMID: 19035781 Free PMC article.
-
Electrochemistry of Alzheimer Disease Amyloid Beta Peptides.Curr Med Chem. 2018;25(33):4066-4083. doi: 10.2174/0929867325666180214112536. Curr Med Chem. 2018. PMID: 29446720 Review.
-
Effects of Cu(II) on the aggregation of amyloid-β.J Biol Inorg Chem. 2019 Dec;24(8):1197-1215. doi: 10.1007/s00775-019-01727-5. Epub 2019 Oct 10. J Biol Inorg Chem. 2019. PMID: 31602542 Review.
Cited by
-
Amyloid plaques beyond Aβ: a survey of the diverse modulators of amyloid aggregation.Biophys Rev. 2017 Aug;9(4):405-419. doi: 10.1007/s12551-017-0271-9. Epub 2017 Jun 19. Biophys Rev. 2017. PMID: 28631243 Free PMC article. Review.
-
ESEEM analysis of multi-histidine Cu(II)-coordination in model complexes, peptides, and amyloid-β.J Phys Chem B. 2014 Jul 31;118(30):8935-44. doi: 10.1021/jp500767n. Epub 2014 Jul 22. J Phys Chem B. 2014. PMID: 25014537 Free PMC article.
-
Cu and Zn coordination to amyloid peptides: From fascinating chemistry to debated pathological relevance.Coord Chem Rev. 2018 Sep 15;375:38-55. doi: 10.1016/j.ccr.2018.04.007. Coord Chem Rev. 2018. PMID: 30262932 Free PMC article.
-
Copper(II) Can Kinetically Trap Arctic and Italian Amyloid-β40 as Toxic Oligomers, Mimicking Cu(II) Binding to Wild-Type Amyloid-β42: Implications for Familial Alzheimer's Disease.JACS Au. 2024 Feb 6;4(2):578-591. doi: 10.1021/jacsau.3c00687. eCollection 2024 Feb 26. JACS Au. 2024. PMID: 38425915 Free PMC article.
-
Design and implementation of an FPGA-based timing pulse programmer for pulsed-electron paramagnetic resonance applications.Concepts Magn Reson Part B Magn Reson Eng. 2013 Aug 1;43(3):100-109. doi: 10.1002/cmr.b.21240. Concepts Magn Reson Part B Magn Reson Eng. 2013. PMID: 25076864 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

