Local structure and global patterning of Cu2+ binding in fibrillar amyloid-β [Aβ(1-40)] protein
- PMID: 23043377
- PMCID: PMC3722434
- DOI: 10.1021/ja306946q
Local structure and global patterning of Cu2+ binding in fibrillar amyloid-β [Aβ(1-40)] protein
Abstract
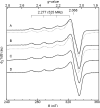
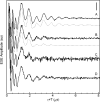
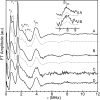
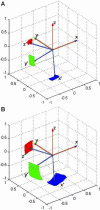
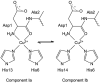
The amyloid-β (Aβ) protein forms fibrils and higher-order plaque aggegrates in Alzheimer's disease (AD) brain. The copper ion, Cu(2+), is found at high concentrations in plaques, but its role in AD etiology is unclear. We use high-resolution pulsed electron paramagnetic resonance spectroscopy to characterize the coordination structure of Cu(2+) in the fibrillar form of full-length Aβ(1-40). The results reveal a bis-cis-histidine (His) equatorial Cu(2+) coordination geometry and participation of all three N-terminal His residues in Cu(2+) binding. A model is proposed in which Cu(2+)-His6/His13 and Cu(2+)-His6/His14 sites alternate along the fibril axis on opposite sides of the β-sheet fibril structure. The local intra-β-strand coordination structure is not conducive to Cu(2+)/Cu(+) redox-linked coordination changes, and the global arrangement of Cu sites precludes facile multielectron and bridged-metal site reactivity. This indicates that the fibrillar form of Aβ suppresses Cu redox cycling and reactive oxygen species production. The configuration suggests application of Cu(2+)-Aβ fibrils as an amyloid architecture for switchable electron charge/spin coupling and redox reactivity.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

