Creating order from chaos: cellular regulation by kinase anchoring
- PMID: 23043438
- PMCID: PMC3540170
- DOI: 10.1146/annurev-pharmtox-011112-140204
Creating order from chaos: cellular regulation by kinase anchoring
Abstract
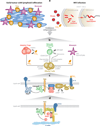
Second messenger responses rely on where and when the enzymes that propagate these signals become active. Spatial and temporal organization of certain signaling enzymes is controlled in part by A-kinase anchoring proteins (AKAPs). This family of regulatory proteins was originally classified on the basis of their ability to compartmentalize the cyclic adenosine monophosphate (cAMP)-dependent protein kinase (also known as protein kinase A, or PKA). However, it is now recognized that AKAPs position G protein-coupled receptors, adenylyl cyclases, G proteins, and their effector proteins in relation to protein kinases and signal termination enzymes such as phosphodiesterases and protein phosphatases. This arrangement offers a simple and efficient means to limit the scope, duration, and directional flow of information to sites deep within the cell. This review focuses on the pros and cons of reagents that define the biological role of kinase anchoring inside cells and discusses recent advances in our understanding of anchored second messenger signaling in the cardiovascular and immune systems.
Figures



References
-
- Rall TW, Sutherland EW, Berthet J. The relationships of epinephrine and glucagon to liver phosphorylase. J. Biol. Chem. 1957;224:463–475. - PubMed
-
- Sutherland EW. Studies on the mechanism of hormone action. Science. 1972;171:401–408. - PubMed
-
- Fischer EH, Krebs EG. Conversion of phosphorylase b to phosphorylase a in muscle extracts. J. Biol. Chem. 1955;216:121–132. - PubMed
-
- Corbin JD, Soderling TR, Park CR. Regulation of adenosine 3′,5′-monophosphate-dependent protein kinase. J. Biol. Chem. 1973;248:1813–1821. - PubMed
-
- Potter RL, Stafford PH, Taylor S. Regulatory subunit of cyclic AMP-dependent protein kinase I from porcine skeletal muscle: purification and proteolysis. Arch. Biochem. Biophys. 1978;190:174–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

