Roles of proteolysis in regulation of GPCR function
- PMID: 23043558
- PMCID: PMC3579280
- DOI: 10.1111/j.1476-5381.2012.02234.x
Roles of proteolysis in regulation of GPCR function
Abstract
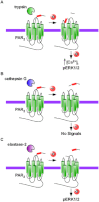
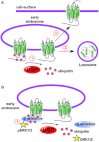
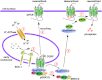
The enzymatic activity of peptidases must be tightly regulated to prevent uncontrolled hydrolysis of peptide bonds, which could have devastating effects on biological systems. Peptidases are often generated as inactive propeptidases, secreted with endogenous inhibitors, or they are compartmentalized. Propeptidases become active after proteolytic removal of N-terminal activation peptides by other peptidases. Some peptidases only become active towards substrates only at certain pHs, thus confining activity to specific compartments or conditions. This review discusses the different roles proteolysis plays in regulating GPCRs. At the cell-surface, certain GPCRs are regulated by the hydrolytic inactivation of bioactive peptides by membrane-anchored peptidases, which prevent signalling. Conversely, cell-surface peptidases can also generate bioactive peptides, which directly activate GPCRs. Alternatively, cell-surface peptidases activated by GPCRs, can generate bioactive peptides to cause transactivation of receptor tyrosine kinases, thereby promoting signalling. Certain peptidases can signal directly to cells, by cleaving GPCR to initiate intracellular signalling cascades. Intracellular peptidases also regulate GPCRs; lysosomal peptidases destroy GPCRs in lysosomes to permanently terminate signalling and mediate down-regulation; endosomal peptidases cleave internalized peptide agonists to regulate GPCR recycling, resensitization and signalling; and soluble intracellular peptidases also participate in GPCR function by regulating the ubiquitination state of GPCRs, thereby altering GPCR signalling and fate. Although the use of peptidase inhibitors has already brought success in the treatment of diseases such as hypertension, the discovery of new regulatory mechanisms involving proteolysis that control GPCRs may provide additional targets to modulate dysregulated GPCR signalling in disease.
© 2012 The Author. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures

References
-
- Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8:35–40. - PubMed
-
- Bennett VJ, Perrine SA, Simmons MA. A novel mechanism of neurokinin-1 receptor resensitization. J Pharmacol Exp Ther. 2002;303:1155–1162. - PubMed
-
- Bennett VJ, Perrine SA, Simmons MA. Neurokinin-1 receptor resensitization precedes receptor recycling. J Pharmacol Exp Ther. 2005;313:1347–1354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

