Histo-anatomical structure of the living isolated rat heart in two contraction states assessed by diffusion tensor MRI
- PMID: 23043978
- PMCID: PMC3526796
- DOI: 10.1016/j.pbiomolbio.2012.07.014
Histo-anatomical structure of the living isolated rat heart in two contraction states assessed by diffusion tensor MRI
Abstract
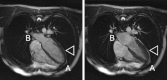
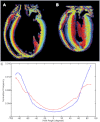
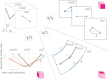
Deformation and wall-thickening of ventricular myocardium are essential for cardiac pump function. However, insight into the histo-anatomical basis for cardiac tissue re-arrangement during contraction is limited. In this report, we describe dynamic changes in regionally prevailing cardiomyocyte (fibre) and myolaminar (sheet) orientations, using Diffusion Tensor Imaging (DTI) of ventricles in the same living heart in two different mechanical states. Hearts, isolated from Sprague-Dawley rats, were Langendorff-perfused and imaged, initially in their slack state during cardioplegic arrest, then during lithium-induced contracture. Regional fibre- and sheet-orientations were derived from DTI-data on a voxel-wise basis. Contraction was accompanied with a decrease in left-handed helical fibres (handedness relative to the baso-apical direction) in basal, equatorial, and apical sub-epicardium (by 14.0%, 17.3%, 15.8% respectively; p < 0.001), and an increase in right-handed helical fibres of the sub-endocardium (by 11.0%, 12.1% and 16.1%, respectively; p < 0.001). Two predominant sheet-populations were observed, with sheet-angles of either positive (β+) or negative (β-) polarity relative to a 'chamber-horizontal plane' (defined as normal to the left ventricular long-axis). In contracture, mean 'intersection'-angle (geometrically quantifiable intersection of sheet-angle projections) between β+ and β- sheet-populations increased from 86.2 ± 5.5° (slack) to 108.3 ± 5.4° (p < 0.001). Subsequent high-resolution DTI of fixed myocardium, and histological sectioning, reconfirmed the existence of alternating sheet-plane populations. Our results suggest that myocardial tissue layers in alternating sheet-populations align into a more chamber-horizontal orientation during contraction. This re-arrangement occurs via an accordion-like mechanism that, combined with inter-sheet slippage, can significantly contribute to ventricular deformation, including wall-thickening in a predominantly centripetal direction and baso-apical shortening.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures




References
-
- Arts T., Costa K.D., Covell J.W., McCulloch A.D. Relating myocardial laminar architecture to shear strain and muscle fiber orientation. Am. J. Physiol. Heart Circ. Physiol. 2001;280(5):H2222–H2229. - PubMed
-
- Basser P.J., Mattiello J., LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B. 1994;103:247–254. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

