Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth
- PMID: 23045606
- PMCID: PMC3478934
- DOI: 10.1084/jem.20111497
Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth
Abstract
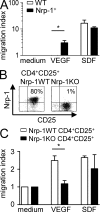
Infiltration of Foxp3(+) regulatory T (T reg) cells is considered to be a critical step during tumor development and progression. T reg cells supposedly suppress locally an effective anti-tumor immune response within tumor tissues, although the precise mechanism by which T reg cells infiltrate the tumor is still unclear. We provide evidence that Neuropilin 1 (Nrp-1), highly expressed by Foxp3(+) T reg cells, regulates the immunological anti-tumor control by guiding T reg cells into the tumor in response to tumor-derived vascular endothelial growth factor (VEGF). We demonstrate for the first time that T cell-specific ablation of Nrp-1 expression results in a significant breakdown in tumor immune escape in various transplantation models and in a spontaneous, endogenously driven melanoma model associated with strongly reduced tumor growth and prolonged tumor-free survival. Strikingly, numbers of tumor-infiltrating Foxp3(+) T reg cells were significantly reduced accompanied by enhanced activation of CD8(+) T cells within tumors of T cell-specific Nrp-1-deficient mice. This phenotype can be reversed by adoptive transfer of Nrp-1(+) T reg cells from wild-type mice. Thus, our data strongly suggest that Nrp-1 acts as a key mediator of Foxp3(+) T reg cell infiltration into the tumor site resulting in a dampened anti-tumor immune response and enhanced tumor progression.
Figures





References
-
- Antony P.A., Piccirillo C.A., Akpinarli A., Finkelstein S.E., Speiss P.J., Surman D.R., Palmer D.C., Chan C.C., Klebanoff C.A., Overwijk W.W., et al. 2005. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 174:2591–2601 - PMC - PubMed
-
- Battaglia A., Buzzonetti A., Monego G., Peri L., Ferrandina G., Fanfani F., Scambia G., Fattorossi A. 2008. Neuropilin-1 expression identifies a subset of regulatory T cells in human lymph nodes that is modulated by preoperative chemoradiation therapy in cervical cancer. Immunology. 123:129–138 10.1111/j.1365-2567.2007.02737.x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

