A Syrian golden hamster model recapitulating ebola hemorrhagic fever
- PMID: 23045629
- PMCID: PMC3532827
- DOI: 10.1093/infdis/jis626
A Syrian golden hamster model recapitulating ebola hemorrhagic fever
Abstract
Ebola hemorrhagic fever (EHF) is a severe viral infection for which no effective treatment or vaccine is currently available. While the nonhuman primate (NHP) model is used for final evaluation of experimental vaccines and therapeutic efficacy, rodent models have been widely used in ebolavirus research because of their convenience. However, the validity of rodent models has been questioned given their low predictive value for efficacy testing of vaccines and therapeutics, a result of the inconsistent manifestation of coagulopathy seen in EHF. Here, we describe a lethal Syrian hamster model of EHF using mouse-adapted Ebola virus. Infected hamsters displayed most clinical hallmarks of EHF, including severe coagulopathy and uncontrolled host immune responses. Thus, the hamster seems to be superior to the existing rodent models, offering a better tool for understanding the critical processes in pathogenesis and providing a new model for evaluating prophylactic and postexposure interventions prior to testing in NHPs.
Figures

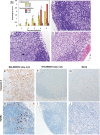


References
-
- Sanchez A, Geisbert T, Feldmann H. Filoviridae: Marburg and Ebola viruses. In: Knipe D, Howley P, editors. Fields virology. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 1409–48.
-
- Geisbert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, Hensley LE. Mechanisms underlying coagulation abnormalities in Ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J Infect Dis. 2003;188:1618–29. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

