The chromatin insulator CTCF and the emergence of metazoan diversity
- PMID: 23045651
- PMCID: PMC3491479
- DOI: 10.1073/pnas.1111941109
The chromatin insulator CTCF and the emergence of metazoan diversity
Abstract
The great majority of metazoans belong to bilaterian phyla. They diversified during a short interval in Earth's history known as the Cambrian explosion, ~540 million years ago. However, the genetic basis of these events is poorly understood. Here we argue that the vertebrate genome organizer CTCF (CCCTC-binding factor) played an important role for the evolution of bilaterian animals. We provide evidence that the CTCF protein and a genome-wide abundance of CTCF-specific binding motifs are unique to bilaterian phyla, but absent in other eukaryotes. We demonstrate that CTCF-binding sites within vertebrate and Drosophila Hox gene clusters have been maintained for several hundred million years, suggesting an ancient origin of the previously known interaction between Hox gene regulation and CTCF. In addition, a close correlation between the presence of CTCF and Hox gene clusters throughout the animal kingdom suggests conservation of the Hox-CTCF link across the Bilateria. On the basis of these findings, we propose the existence of a Hox-CTCF kernel as principal organizer of bilaterian body plans. Such a kernel could explain (i) the formation of Hox clusters in Bilateria, (ii) the diversity of bilaterian body plans, and (iii) the uniqueness and time of onset of the Cambrian explosion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
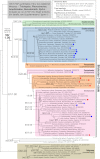

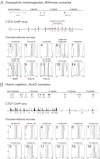

References
-
- Canfield DE, Poulton SW, Narbonne GM. Late-Neoproterozoic deep-ocean oxygenation and the rise of animal life. Science. 2007;315:92–95. - PubMed
-
- Marshall CR. Explaining the Cambrian “explosion” of animals. Annu Rev Earth Planet Sci. 2006;34:355–384.
-
- Putnam NH, et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

