A composite bacteriophage alters colonization by an intestinal commensal bacterium
- PMID: 23045666
- PMCID: PMC3491505
- DOI: 10.1073/pnas.1206136109
A composite bacteriophage alters colonization by an intestinal commensal bacterium
Abstract
The mammalian intestine is home to a dense community of bacteria and its associated bacteriophage (phage). Virtually nothing is known about how phages impact the establishment and maintenance of resident bacterial communities in the intestine. Here, we examine the phages harbored by Enterococcus faecalis, a commensal of the human intestine. We show that E. faecalis strain V583 produces a composite phage (ΦV1/7) derived from two distinct chromosomally encoded prophage elements. One prophage, prophage 1 (ΦV1), encodes the structural genes necessary for phage particle production. Another prophage, prophage 7 (ΦV7), is required for phage infection of susceptible host bacteria. Production of ΦV1/7 is controlled, in part, by nutrient availability, because ΦV1/7 particle numbers are elevated by free amino acids in culture and during growth in the mouse intestine. ΦV1/7 confers an advantage to E. faecalis V583 during competition with other E. faecalis strains in vitro and in vivo. Thus, we propose that E. faecalis V583 uses phage particles to establish and maintain dominance of its intestinal niche in the presence of closely related competing strains. Our findings indicate that bacteriophages can impact the dynamics of bacterial colonization in the mammalian intestinal ecosystem.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
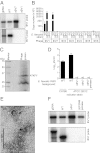
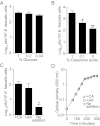


Comment in
-
Prophage: a crucial catalyst in infectious disease modulation.Lancet Microbe. 2022 Mar;3(3):e162-e163. doi: 10.1016/S2666-5247(21)00354-2. Epub 2022 Jan 25. Lancet Microbe. 2022. PMID: 35544071 No abstract available.
References
-
- Duerkop BA, Vaishnava S, Hooper LV. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31:368–376. - PubMed
-
- Fischetti VA. In vivo acquisition of prophage in Streptococcus pyogenes. Trends Microbiol. 2007;15:297–300. - PubMed
-
- Hambly E, Suttle CA. The viriosphere, diversity, and genetic exchange within phage communities. Curr Opin Microbiol. 2005;8:444–450. - PubMed
-
- Rodriguez-Valera F, et al. Explaining microbial population genomics through phage predation. Nat Rev Microbiol. 2009;7:828–836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

