Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy
- PMID: 23045683
- PMCID: PMC3491458
- DOI: 10.1073/pnas.1215397109
Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy
Abstract
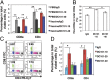
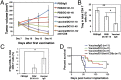
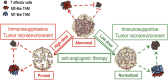
The recent approval of a prostate cancer vaccine has renewed hope for anticancer immunotherapies. However, the immunosuppressive tumor microenvironment may limit the effectiveness of current immunotherapies. Antiangiogenic agents have the potential to modulate the tumor microenvironment and improve immunotherapy, but they often are used at high doses in the clinic to prune tumor vessels and paradoxically may compromise various therapies. Here, we demonstrate that targeting tumor vasculature with lower vascular-normalizing doses, but not high antivascular/antiangiogenic doses, of an anti-VEGF receptor 2 (VEGFR2) antibody results in a more homogeneous distribution of functional tumor vessels. Furthermore, lower doses are superior to the high doses in polarizing tumor-associated macrophages from an immune inhibitory M2-like phenotype toward an immune stimulatory M1-like phenotype and in facilitating CD4(+) and CD8(+) T-cell tumor infiltration. Based on this mechanism, scheduling lower-dose anti-VEGFR2 therapy with T-cell activation induced by a whole cancer cell vaccine therapy enhanced anticancer efficacy in a CD8(+) T-cell-dependent manner in both immune-tolerant and immunogenic murine breast cancer models. These findings indicate that vascular-normalizing lower doses of anti-VEGFR2 antibody can reprogram the tumor microenvironment away from immunosuppression toward potentiation of cancer vaccine therapies. Given that the combinations of high doses of bevacizumab with chemotherapy have not improved overall survival of breast cancer patients, our study suggests a strategy to use antiangiogenic agents in breast cancer more effectively with active immunotherapy and potentially other anticancer therapies.
Conflict of interest statement
Conflict of interest statement: R.K.J. received research grants from Dyax, MedImmune, and Roche; consultant fees from Dyax, Enlight, Noxxon, and SynDevRx; owns equity in Enlight, SynDevRx, and XTuit; and serves on the Board of Directors of XTuit and Boards of Trustees of H&Q Healthcare Investors and H&Q Life Sciences Investors. M.C.P. serves as a scientific adviser to Evaxion-Biotech and owns equity in Celtaxsys. No reagents or funding from these companies was used in this study. Therefore, there is no significant financial or other competing interest in the work.
Figures
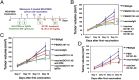
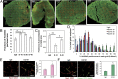
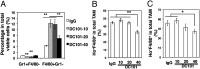
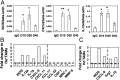
References
-
- Rosenberg SA, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. - PubMed
-
- Offringa R. Cancer. Cancer immunotherapy is more than a numbers game. Science. 2006;314:68–69. - PubMed
-
- Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA126642/CA/NCI NIH HHS/United States
- R01 CA149285/CA/NCI NIH HHS/United States
- R01-CA115767/CA/NCI NIH HHS/United States
- R01-CA096915/CA/NCI NIH HHS/United States
- R01 CA159258/CA/NCI NIH HHS/United States
- R01 CA115767/CA/NCI NIH HHS/United States
- R01 CA096915/CA/NCI NIH HHS/United States
- R01 HL106584/HL/NHLBI NIH HHS/United States
- R01-CA159258/CA/NCI NIH HHS/United States
- R21 CA139168/CA/NCI NIH HHS/United States
- P01 CA080124/CA/NCI NIH HHS/United States
- R21-CA139168/CA/NCI NIH HHS/United States
- R01-CA126642/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

