T cells and their eons-old obsession with MHC
- PMID: 23046122
- PMCID: PMC3963424
- DOI: 10.1111/imr.12004
T cells and their eons-old obsession with MHC
Abstract
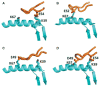
T cells bearing receptors made up of α and β chains (TCRs) usually react with peptides bound to major histocompatibility complex proteins (MHC). This bias could be imposed by positive selection, the phenomenon that selects thymocytes to mature into T cells only if the TCRs they bear react with low but appreciable affinity with MHC + peptide combinations in the thymus cortex. However, it is also possible that the polypeptides of TCRs themselves do not have random specificities but rather are biased toward reaction with MHC. Evolution would therefore have selected for a collection of TCR variable elements that are prone to react with MHC. If this were to be so, positive selection would act on thymocytes bearing a pre biased collection of TCRs to pick out those that react to some extent, but not too well, with self MHC + self-peptides. A problem with studies of this evolutionary idea is the fact that there are many TCR variable elements and that these differ considerably in the amino acids with which they contact MHC. However, recent experiments by our group and others suggest that one group of TCR variable elements, those related to the mouse Vβ8 family, has amino acids in their CDR2 regions that consistently bind a particular site on an MHC α-helix. Other groups of variable elements may use different patterns of amino acids to achieve the same goal. Mutation of these amino acids reduces the ability of T cells and thymocytes to react with MHC. These amino acids are present in the variable regions of distantly related species such as sharks and human. Overall the data indicate that TCR elements have indeed been selected by evolution to react with MHC proteins. Many mysteries about TCRs remain to be solved, including the nature of auto-recognition, the basis of MHC allele specificity, and the very nature and complexity of TCRs on mature T cells.
© 2012 John Wiley & Sons A/S.
Figures



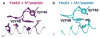


References
-
- The Nobel Lectures in Immunology. The Nobel Prize for Physiology or Medicine, 1980 awarded to Baruj Benacerraf, Jean Daussett & George D. Snell. Scand J Immunol. 1992;35:373–398. - PubMed
-
- Gorer P. The antigenic structure of tumors. Adv Immunol. 1961;1:345.
-
- Babbitt BP, Allen PM, Matsueda G, Haber E, Unanue ER. Binding of immunogenic peptides to Ia histocompatibility molecules. Nature. 1985;317:359–361. - PubMed
-
- Benacerraf B, McDevitt HO. Histocompatibility-linked immune response genes. Science. 1972;175:273–279. - PubMed
-
- Bevan MJ. Interaction antigens detected by cytotoxic T cells with the major histocompatibility complex as modifier. Nature. 1975;256:419–421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI018785/AI/NIAID NIH HHS/United States
- AI-078246/AI/NIAID NIH HHS/United States
- T32 AI007405/AI/NIAID NIH HHS/United States
- AI-18785/AI/NIAID NIH HHS/United States
- R37 AI018785/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R21 AI076463/AI/NIAID NIH HHS/United States
- R56 AI078246/AI/NIAID NIH HHS/United States
- AI-076463/AI/NIAID NIH HHS/United States
- AI-22295/AI/NIAID NIH HHS/United States
- R56 AI018785/AI/NIAID NIH HHS/United States
- P01 AI022295/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

