p38β MAPK upregulates atrogin1/MAFbx by specific phosphorylation of C/EBPβ
- PMID: 23046544
- PMCID: PMC3534568
- DOI: 10.1186/2044-5040-2-20
p38β MAPK upregulates atrogin1/MAFbx by specific phosphorylation of C/EBPβ
Abstract
Background: The p38 mitogen-activated protein kinases (MAPK) family plays pivotal roles in skeletal muscle metabolism. Recent evidence revealed that p38α and p38β exert paradoxical effects on muscle protein homeostasis. However, it is unknown why p38β, but not p38α, is capable of mediating muscle catabolism via selective activation of the C/EBPβ that upregulates atrogin1/MAFbx.
Methods: Tryptic phosphopeptide mapping was carried out to identify p38α- and p38β-mediated phosphorylation sites in C/EBPβ. Chromosome immunoprecipitation (ChIP) assay was used to evaluate p38α and p38β effect on C/EBPβ binding to the atrogin1/MAFbx promoter. Overexpression or siRNA-mediated gene knockdown of p38α and p38β, and site-directed mutagenesis or knockout of C/EBPβ, were used to analyze the roles of these kinases in muscle catabolism in C2C12 myotubes and mice.
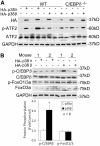
Results: Cellular expression of constitutively active p38α or p38β resulted in phosphorylation of C/EBPβ at multiple serine and threonine residues; however, only p38β phosphorylated Thr-188, which had been known to be critical to the DNA-binding activity of C/EBPβ. Only p38β, but not p38α, activated C/EBPβ-binding to the atrogin1/MAFbx promoter. A C/EBPβ mutant in which Thr-188 was replaced by alanine acted as a dominant-negative inhibitor of atrogin1/MAFbx upregulation induced by either p38β or Lewis lung carcinoma (LLC) cell-conditioned medium (LCM). In addition, knockdown of p38β specifically inhibited C/EBPβ activation and atrogin1/MAFbx upregulation induced by LCM. Finally, expression of active p38β in mouse tibialis anterior specifically induced C/EBPβ phosphorylation at Thr-188, atrogin1/MAFbx upregulation and muscle mass loss, which were blocked in C/EBPβ-null mice.
Conclusions: The α and β isoforms of p38 MAPK are capable of recognizing distinct phosphorylation sites in a substrate. The unique capacity of p38β in mediating muscle catabolism is due to its capability in phosphorylating Thr-188 of C/EBPβ.
Figures


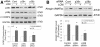

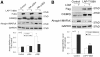

References
Grants and funding
LinkOut - more resources
Full Text Sources

