Pathways of arsenic uptake and efflux
- PMID: 23046656
- PMCID: PMC4578627
- DOI: 10.1016/B978-0-12-394390-3.00012-4
Pathways of arsenic uptake and efflux
Abstract
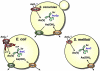
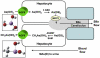
Arsenic is the most prevalent environmental toxic substance and ranks first on the U.S. Environmental Protection Agency's Superfund List. Arsenic is a carcinogen and a causative agent of numerous human diseases. Paradoxically arsenic is used as a chemotherapeutic agent for treatment of acute promyelocytic leukemia. Inorganic arsenic has two biological important oxidation states: As(V) (arsenate) and As(III) (arsenite). Arsenic uptake is adventitious because the arsenate and arsenite are chemically similar to required nutrients. Arsenate resembles phosphate and is a competitive inhibitor of many phosphate-utilizing enzymes. Arsenate is taken up by phosphate transport systems. In contrast, at physiological pH, the form of arsenite is As(OH)(3), which resembles organic molecules such as glycerol. Consequently, arsenite is taken into cells by aquaglyceroporin channels. Arsenic efflux systems are found in nearly every organism and evolved to rid cells of this toxic metalloid. These efflux systems include members of the multidrug resistance protein family and the bacterial exchangers Acr3 and ArsB. ArsB can also be a subunit of the ArsAB As(III)-translocating ATPase, an ATP-driven efflux pump. The ArsD metallochaperone binds cytosolic As(III) and transfers it to the ArsA subunit of the efflux pump. Knowledge of the pathways and transporters for arsenic uptake and efflux is essential for understanding its toxicity and carcinogenicity and for rational design of cancer chemotherapeutic drugs.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Aaltonen EK, Silow M. Transmembrane topology of the Acr3 family arsenite transporter from Bacillus subtilis. Biochimica et Biophysica Acta. 2008;1778(4):963–973. - PubMed
-
- Abernathy CO, Thomas DJ, Calderon RL. Health effects and risk assessment of arsenic. Journal of Nutrition. 2003;133(5 Suppl. 1):1536S–1538S. - PubMed
-
- Achour AR, Bauda P, Billard P. Diversity of arsenite transporter genes from arsenic-resistant soil bacteria. Research in Microbiology. 2007;158(2):128–137. - PubMed
-
- Agre P. Nobel lecture. Aquaporin water channels. Bioscience Reports. 2004;24(3):127–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

