Normal glucose uptake in the brain and heart requires an endothelial cell-specific HIF-1α-dependent function
- PMID: 23047702
- PMCID: PMC3491491
- DOI: 10.1073/pnas.1209281109
Normal glucose uptake in the brain and heart requires an endothelial cell-specific HIF-1α-dependent function
Abstract
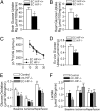
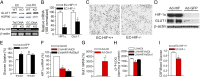
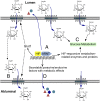
Although intimately positioned between metabolic substrates in the bloodstream and the tissue parenchymal cells that require these substrates, a major role of the vascular endothelium in the regulation of tissue metabolism has not been widely appreciated. We hypothesized that via control of transendothelial glucose transport and contributing paracrine mechanisms the endothelium plays a major role in regulating organ and tissue glucose metabolism. We further hypothesized that the hypoxia-inducible factor -1α (HIF-1α) plays an important role in coordinating these endothelial functions. To test these hypotheses, we generated mice with endothelial cell-specific deletion of HIF-1α. Loss of HIF in the endothelium resulted in significantly increased fasting blood glucose levels, a blunted insulin response with delayed glucose clearance from the blood after i.v. loading, and significantly decreased glucose uptake into the brain and heart. Endothelial HIF-1α knockout mice also exhibited a reduced cerebrospinal fluid/blood glucose ratio, a finding consistent with reduced transendothelial glucose transport and a diagnostic criterion for the Glut1 deficiency genetic syndrome. Endothelial cells from these mice demonstrated decreased Glut1 levels and reduced glucose uptake that was reversed by forced expression of Glut1. These data strongly support an important role of the vascular endothelium in determining whole-organ glucose metabolism and indicate that HIF-1α is a critical mediator of this function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Westermeier F, et al. Equilibrative nucleoside transporters in fetal endothelial dysfunction in diabetes mellitus and hyperglycaemia. Curr Vasc Pharmacol. 2009;7(4):435–449. - PubMed
-
- Seidner G, et al. GLUT-1 deficiency syndrome caused by haploinsufficiency of the blood-brain barrier hexose carrier. Nat Genet. 1998;18(2):188–191. - PubMed
-
- Wang D, et al. Glut-1 deficiency syndrome: Clinical, genetic, and therapeutic aspects. Ann Neurol. 2005;57(1):111–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

