Heparanase enhances the insulin receptor signaling pathway to activate extracellular signal-regulated kinase in multiple myeloma
- PMID: 23048032
- PMCID: PMC3510827
- DOI: 10.1074/jbc.M112.391417
Heparanase enhances the insulin receptor signaling pathway to activate extracellular signal-regulated kinase in multiple myeloma
Abstract
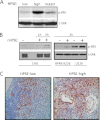
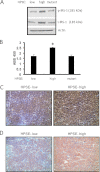
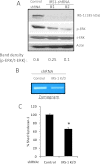
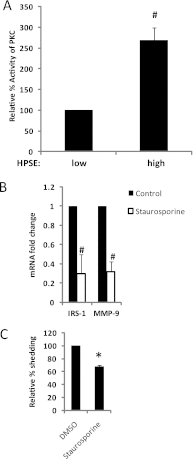
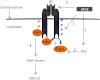
ERK signaling regulates proliferation, survival, drug resistance, and angiogenesis in cancer. Although the mechanisms regulating ERK activation are not fully understood, we previously demonstrated that ERK phosphorylation is elevated by heparanase, an enzyme associated with aggressive behavior of many cancers. In the present study, myeloma cell lines expressing either high or low levels of heparanase were utilized to determine how heparanase stimulates ERK signaling. We discovered that the insulin receptor was abundant on cells expressing either high or low levels of heparanase, but the receptor was highly phosphorylated in heparanase-high cells compared with heparanase-low cells. In addition, protein kinase C activity was elevated in heparanase-high cells, and this enhanced expression of insulin receptor substrate-1 (IRS-1), the principle intracellular substrate for phosphorylation by the insulin receptor. Blocking insulin receptor function with antibody or a small molecule inhibitor or knockdown of IRS-1 expression using shRNA diminished heparanase-mediated ERK activation in the tumor cells. In addition, up-regulation of the insulin signaling pathway by heparanase and the resulting ERK activation were dependent on heparanase retaining its enzyme activity. These results reveal a novel mechanism whereby heparanase enhances activation of the insulin receptor signaling pathway leading to ERK activation and modulation of myeloma behavior.
Figures


References
-
- Ilan N., Elkin M., Vlodavsky I. (2006) Regulation, function, and clinical significance of heparanase in cancer metastasis and angiogenesis. Int. J. Biochem. Cell Biol. 38, 2018–2039 - PubMed
-
- Sanderson R. D., Yang Y., Suva L. J., Kelly T. (2004) Heparan sulfate proteoglycans and heparanase–partners in osteolytic tumor growth and metastasis. Matrix Biol. 23, 341–352 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

