Maternal and neonatal circulating markers of metabolic and cardiovascular risk in the metformin in gestational diabetes (MiG) trial: responses to maternal metformin versus insulin treatment
- PMID: 23048188
- PMCID: PMC3579335
- DOI: 10.2337/dc12-1097
Maternal and neonatal circulating markers of metabolic and cardiovascular risk in the metformin in gestational diabetes (MiG) trial: responses to maternal metformin versus insulin treatment
Abstract
Objective: This study was designed to compare glucose, lipids, and C-reactive protein (CRP) in women with gestational diabetes mellitus treated with metformin or insulin and in cord plasma of their offspring and to examine how these markers relate to infant size at birth.
Research design and methods: Women with gestational diabetes mellitus were randomly assigned to metformin or insulin in the Metformin in Gestational Diabetes trial. Fasting maternal plasma glucose, lipids, and CRP were measured at randomization, 36 weeks' gestation, and 6-8 weeks postpartum as well as in cord plasma. Women with available cord blood samples (metformin n = 236, insulin n = 242) were included.
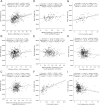
Results: Maternal plasma triglycerides increased more from randomization to 36 weeks' gestation in women treated with metformin (21.93%) versus insulin (9.69%, P < 0.001). Maternal and cord plasma lipids, CRP, and neonatal anthropometry did not differ between treatments. In logistic regression analyses adjusted for confounders, the strongest associations with birth weight >90th centile were maternal triglycerides and measures of glucose control at 36 weeks.
Conclusions: There were few differences in circulating maternal and neonatal markers of metabolic status and no differences in measures of anthropometry between the offspring of women treated with metformin and the offspring of women treated with insulin. There may be subtle effects of metformin on maternal lipid function, but the findings suggest that treating gestational diabetes mellitus with metformin does not adversely affect lipids or CRP in cord plasma or neonatal anthropometric measures.
Figures
Comment in
-
Should metformin be preferred over insulin therapy in the management of gestational diabetes (GDM)?Evid Based Med. 2013 Dec;18(6):e60. doi: 10.1136/eb-2013-101329. Epub 2013 May 21. Evid Based Med. 2013. PMID: 23695953 No abstract available.
References
-
- Hadar E, Hod M. Establishing consensus criteria for the diagnosis of diabetes in pregnancy following the HAPO study. Ann N Y Acad Sci 2010;1205:88–93 - PubMed
-
- Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 - PubMed
-
- Carolan M, Davey MA, Biro MA, Kealy M. Maternal age, ethnicity and gestational diabetes mellitus. Midwifery 2012;28:778–783 - PubMed
-
- Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med 2010;23:199–203 - PubMed
-
- Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773–1779 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

