Deletion of Rictor in neural progenitor cells reveals contributions of mTORC2 signaling to tuberous sclerosis complex
- PMID: 23049074
- PMCID: PMC3522403
- DOI: 10.1093/hmg/dds414
Deletion of Rictor in neural progenitor cells reveals contributions of mTORC2 signaling to tuberous sclerosis complex
Abstract
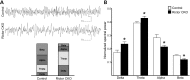
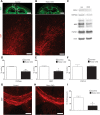
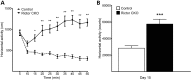
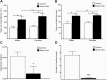
Tuberous sclerosis complex (TSC) is a multisystem genetic disorder with severe neurologic manifestations, including epilepsy, autism, anxiety and attention deficit hyperactivity disorder. TSC is caused by the loss of either the TSC1 or TSC2 genes that normally regulate the mammalian target of rapamycin (mTOR) kinase. mTOR exists within two distinct complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). Loss of either TSC gene leads to increased mTORC1 but decreased mTORC2 signaling. As the contribution of decreased mTORC2 signaling to neural development and homeostasis has not been well studied, we generated a conditional knockout (CKO) of Rictor, a key component of mTORC2. mTORC2 signaling is impaired in the brain, whereas mTORC1 signaling is unchanged. Rictor CKO mice have small brains and bodies, normal lifespan and are fertile. Cortical layering is normal, but neurons are smaller than those in control brains. Seizures were not observed, although excessive slow activity was seen on electroencephalography. Rictor CKO mice are hyperactive and have reduced anxiety-like behavior. Finally, there is decreased white matter and increased levels of monoamine neurotransmitters in the cerebral cortex. Loss of mTORC2 signaling in the cortex independent of mTORC1 can disrupt normal brain development and function and may contribute to some of the neurologic manifestations seen in TSC.
Figures



References
-
- Kim D.H., Sarbassov D.D., Ali S.M., King J.E., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi:10.1016/S0092-8674(02)00808-5. - DOI - PubMed
-
- Liu L.H., Das S., Losert W., Parent C.A. mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev. Cell. 2010;19:845–857. doi:10.1016/j.devcel.2010.11.004. - DOI - PMC - PubMed
-
- Fingar D.C., Richardson C.J., Tee A.R., Cheatham L., Tsou C., Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol. Cell Biol. 2004;24:200–216. doi:10.1128/MCB.24.1.200-216.2004. - DOI - PMC - PubMed
-
- Hay N., Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi:10.1101/gad.1212704. - DOI - PubMed
-
- Sarbassov D.D., Ali S.M., Sengupta S., Sheen J.H., Hsu P.P., Bagley A.F., Markhard A.L., Sabatini D.M. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell. 2006;22:159–168. doi:10.1016/j.molcel.2006.03.029. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

