Characteristics and management of immunerelated adverse effects associated with ipilimumab, a new immunotherapy for metastatic melanoma
- PMID: 23049279
- PMCID: PMC3459593
- DOI: 10.2147/CMAR.S31873
Characteristics and management of immunerelated adverse effects associated with ipilimumab, a new immunotherapy for metastatic melanoma
Abstract
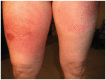
When diagnosed in its early stages, melanoma is highly treatable and associated with good long-term outcomes; however, the prognosis is much poorer for patients diagnosed with advanced or metastatic melanoma. For decades, available treatments were effective in only a few patients and associated with significant safety concerns. Ipilimumab is a novel immunotherapy which has proved to be an exciting breakthrough in the treatment of melanoma. It is the first drug approved for the treatment of melanoma by the Food and Drug Administration (FDA) which has shown a survival benefit in a randomized Phase III clinical trial. The objective of this review is to provide information on the administration, treatment responses, and expected outcomes of treatment of metastatic melanoma with the new immunotherapeutic agent, ipilimumab, a drug with a unique mechanism of action that differentiates it from current treatments. Guidelines for the management of immune-related adverse events associated with ipilimumab therapy are also presented. These stress vigilance, prompt intervention, and the use of corticosteroids as appropriate. Various ipilimumab-associated immune-related adverse events, both common (enterocolitis, dermatitis) and less frequent (hepatitis, hypophysitis), are illustrated in case studies. Nurses are uniquely positioned to provide patient and caregiver education on how this new therapy differs from traditional cytotoxic agents, to recognize the signs and symptoms of immune-related adverse events, and to report them immediately, and finally, to be aware of the patterns of response that are commonly observed in patients receiving ipilimumab therapy.
Keywords: case studies; immunotherapy; ipilimumab; melanoma.
Figures
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. - PubMed
-
- Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review 1975–2008. National Cancer Institute; Bethesda, MD: [Accessed September 20, 2011]. Available from: http://seer.cancer.gov/csr/1975_2008/
-
- National Comprehensive Cancer Network. Melanoma. 2012. [Accessed October 28, 2011]. Available from: http://www.nccn.org/index.asp.
LinkOut - more resources
Full Text Sources

