Photovoltaic Retinal Prosthesis with High Pixel Density
- PMID: 23049619
- PMCID: PMC3462820
- DOI: 10.1038/nphoton.2012.104
Photovoltaic Retinal Prosthesis with High Pixel Density
Abstract
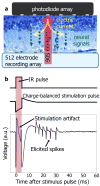
Retinal degenerative diseases lead to blindness due to loss of the "image capturing" photoreceptors, while neurons in the "image processing" inner retinal layers are relatively well preserved. Electronic retinal prostheses seek to restore sight by electrically stimulating surviving neurons. Most implants are powered through inductive coils, requiring complex surgical methods to implant the coil-decoder-cable-array systems, which deliver energy to stimulating electrodes via intraocular cables. We present a photovoltaic subretinal prosthesis, in which silicon photodiodes in each pixel receive power and data directly through pulsed near-infrared illumination and electrically stimulate neurons. Stimulation was produced in normal and degenerate rat retinas, with pulse durations from 0.5 to 4 ms, and threshold peak irradiances from 0.2 to 10 mW/mm(2), two orders of magnitude below the ocular safety limit. Neural responses were elicited by illuminating a single 70 μm bipolar pixel, demonstrating the possibility of a fully-integrated photovoltaic retinal prosthesis with high pixel density.
Figures





References
-
- Smith W, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108:697–704. - PubMed
-
- Haim M. Epidemiology of retinitis pigmentosa in Denmark. Acta Ophthalmol Scand Suppl. 2002:1–34. - PubMed
-
- Kim SY, et al. Morphometric analysis of the macula in eyes with disciform age-related macular degeneration. Retina. 2002;22:471–477. - PubMed
-
- Stone JL, Barlow WE, Humayun MS, de Juan E, Jr, Milam AH. Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch Ophthalmol. 1992;110:1634–1639. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
