Humanization and characterization of an anti-ricin neutralization monoclonal antibody
- PMID: 23049820
- PMCID: PMC3458913
- DOI: 10.1371/journal.pone.0045595
Humanization and characterization of an anti-ricin neutralization monoclonal antibody
Abstract
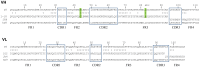
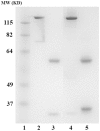
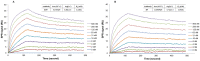
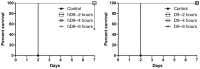
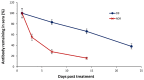
Ricin is regarded as a high terrorist risk for the public due to its high toxicity and ease of production. Currently, there is no therapeutic or vaccine available against ricin. D9, a murine monoclonal antibody developed previously in our laboratory, can strongly neutralize ricin and is therefore a good candidate for humanization. Humanization of D9 variable regions was achieved by a complementarity-determining region grafting approach. The humanized D9 (hD9) variable regions were further grafted onto human heavy and light chain constant regions to assemble the complete antibody gene. A foot-and-mouth-disease virus-derived 2A self-processing sequence was introduced between heavy and light chain DNA sequences to cleave the recombinant protein into a functional full-length antibody molecule from a single open reading frame driven by a single promoter in an adenoviral vector. After expression in mammalian cells and purification, the hD9 was demonstrated to have equimolar expression of the full-length antibody heavy and light chains. More importantly, the hD9 exhibited high affinity to ricin with K(D) of 1.63 nM, comparable to its parental murine D9 (2.55 nM). In a mouse model, intraperitoneal (i.p.) administration of hD9, at a low dose of 5 µg per mouse, 4 hours after the i.p. challenge with 5×LD50 ricin was found to rescue 100% of the mice. In addition, administered 6 hours post-challenge, hD9 could still rescue 50% of the mice. The hD9 has the potential to be used for prophylactic or therapeutic purposes against ricin poisoning.
Conflict of interest statement
Figures
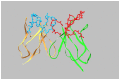


Similar articles
-
Design and Generation of Humanized Single-chain Fv Derived from Mouse Hybridoma for Potential Targeting Application.Monoclon Antib Immunodiagn Immunother. 2015 Dec;34(6):404-17. doi: 10.1089/mab.2015.0036. Monoclon Antib Immunodiagn Immunother. 2015. PMID: 26683180 Free PMC article.
-
Humanized antibody neutralizing 2009 pandemic H1N1 virus.Biotechnol J. 2014 Dec;9(12):1594-603. doi: 10.1002/biot.201400083. Epub 2014 Aug 8. Biotechnol J. 2014. PMID: 25044602
-
Conformation-dependent high-affinity potent ricin-neutralizing monoclonal antibodies.Biomed Res Int. 2013;2013:471346. doi: 10.1155/2013/471346. Epub 2012 Dec 26. Biomed Res Int. 2013. PMID: 23484120 Free PMC article.
-
Understanding the Significance and Implications of Antibody Numbering and Antigen-Binding Surface/Residue Definition.Front Immunol. 2018 Oct 16;9:2278. doi: 10.3389/fimmu.2018.02278. eCollection 2018. Front Immunol. 2018. PMID: 30386328 Free PMC article. Review.
-
Antibody humanization methods - a review and update.Biotechnol Genet Eng Rev. 2013;29:175-86. doi: 10.1080/02648725.2013.801235. Epub 2013 Aug 2. Biotechnol Genet Eng Rev. 2013. PMID: 24568279 Review.
Cited by
-
Active immunity induced by passive IgG post-exposure protection against ricin.Toxins (Basel). 2014 Jan 21;6(1):380-93. doi: 10.3390/toxins6010380. Toxins (Basel). 2014. PMID: 24451844 Free PMC article.
-
A novel recombinant vaccine protecting mice against abrin intoxication.Hum Vaccin Immunother. 2015;11(6):1361-7. doi: 10.1080/21645515.2015.1008879. Hum Vaccin Immunother. 2015. PMID: 26086588 Free PMC article.
-
Structure guided homology model based design and engineering of mouse antibodies for humanization.Bioinformation. 2014 Apr 23;10(4):180-6. doi: 10.6026/97320630010180. eCollection 2014. Bioinformation. 2014. PMID: 24966517 Free PMC article.
-
Humanized Monoclonal Antibody That Passively Protects Mice against Systemic and Intranasal Ricin Toxin Challenge.Clin Vaccine Immunol. 2016 Sep 6;23(9):795-9. doi: 10.1128/CVI.00088-16. Print 2016 Sep. Clin Vaccine Immunol. 2016. PMID: 27466351 Free PMC article.
-
Isolation of Anti-Ricin Protective Antibodies Exhibiting High Affinity from Immunized Non-Human Primates.Toxins (Basel). 2016 Mar 3;8(3):64. doi: 10.3390/toxins8030064. Toxins (Basel). 2016. PMID: 26950154 Free PMC article.
References
-
- Nicolson GL, Lacorbiere M, Hunter TR (1975) Mechanism of cell entry and toxicity of an affinity- purified lectin from Ricinus communis and its differential effects on normal and virus-transformed fibroblasts. Cancer Res 35: 144–155. - PubMed
-
- Youle RJ, Colombatti M (1987) Hybridoma cells containing intracellular anti-ricin antibodies show ricin meets secretory antibody before entering the cytosol. J Biol Chem 262: 4676–4682. - PubMed
-
- Simmons BM, Stahl PD, Russell JH (1986) Mannose receptor-mediated uptake of ricin toxin and ricin A chain by macrophages. Multiple intracellular pathways for a chain translocation. J Biol Chem 261: 7912–7920. - PubMed
-
- Endo Y, Mitsui K, Motizuki M, Tsurugi K (1987) The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem 262: 5908–5912. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

